Introduction
Subarachnoid haemorrhage (SAH) refers to the accumulation of blood within the subarachnoid space. The aetiology of SAH can be broadly classified into 2 categories: traumatic SAH and non-traumatic or spontaneous SAH [1]. Traumatic SAH, which frequently occurs after head trauma, is typically a benign condition that exhibits a favourable prognosis, rarely necessitating surgical intervention [1]. Unlike spontaneous SAH, which has the risk of a life-threatening condition. It affects 6 in 100,000 people annually [2], accounting for 2% to 7% of all strokes [2]. It is associated with up to 45% of 30-day mortality as well as poor functional outcomes in survivors [3]. The most common cause of spontaneous SAH is a ruptured cerebral aneurysm in approximately 85% of cases [4]. Additional aetiologies that might cause SAH include intracranial artery dissection, arteriovenous malformation/fistula, coagulopathy, drug abuse, or cerebral amyloid angiopathy [4]. Therefore, determination of a curable cause of SAH is essential for treatment planning.
It is important to consider the diagnosis of spontaneous SAH in patients who present with symptoms, typically a sudden onset of severe headache. Syncope, vomiting, neck pain, and convulsions are additional symptoms that suggest a subarachnoid bleed. A benign neurologic exa-mination is observed in as many as 50% of patients with SAH, despite the possibility of focal neurologic deficits, meningismus, or retinal haemorrhage [5].
Unenhanced computed tomography (CT) is the initial diagnostic modality when a clinical suspicion for SAH is present based on the patient’s history and physical examination. The patterns of SAH based on blood distribution on CT can be classified in a variety of ways, typically being separated into diffuse, perimesencephalic, and convexal pattern [6]. This simplifies the differential diagnosis and informs the prospect of further imaging. A lumbar puncture should be performed when a CT scan reveals a negative result.
After detecting the presence of SAH, computed tomography angiography (CTA) is typically the next imaging modality used to identify any potential underlying aetiology. When a patient’s CTA result is negative, digital subtraction angiography (DSA), which continues to be the gold standard for lumen-based imaging resolution, is often performed following CTA [7]. The main aetio-logy of spontaneous SAH is a ruptured cerebral aneurysm. However, in about 15% of cases the source of bleeding cannot be determined [8], leading to a condition known as idiopathic SAH.
For imaging follow-up in the initial DSA-negative SAH, there are no universally accepted guidelines. Repeat DSA has its own set of high-risk effects. Neurologic complications were observed in 2.63% of cases, with 0.14% of these cases resulting in strokes followed by permanent disabilities [9]. It is essential to balance the risks against the benefit of repeat DSA. According to a meta-analysis of 40 studies [10], the rate of positive findings after a repeat DSA in patients with perimesencephalic SAH was 1% or less. It could be attributed to major technological advancements in CT equipment and techniques over the past 20 years that have increased diagnostic accuracy. The advanced, modern multidetector CTA has become a valuable, non-invasive alternative to DSA for evaluation without high-risk complications. The yield of repeat CTA for the diagnosis of occult vascular lesions in patients with SAH and negative initial CTA and DSA has also been determined in a small number of publications [6, 11, 12].
In this study, we present a single-centre experience of repeat CTA within a 2-week period after initial DSA negative SAH. The findings will help us to quantify the yield of repeat CTA for detection of occult causative vascular lesion.
Material and methods
Study cohort
This is a retrospective observational cohort study in a single institution, Maharaj Nakorn Chiang Mai Hospital, Chiang Mai University, Thailand. The study was approved by the Ethics Committee of the Faculty of Medicine at Chiang Mai University (Study code: RAD-2565-09234, Research ID 9234).
We reviewed 422 patients with non-traumatic SAHs between January 2013 and July 2022. In total, 422 patients with non-traumatic SAH were examined. Unenhanced CT and CTA were used to diagnose SAH. After diagnosis, patients underwent a cerebral DSA procedure. We diagnosed 52 individuals as having a negative initial DSA and included them in our study. All patients underwent standard follow-up CTA in accordance with the protocol at our institution within 1-2 weeks of a negative initial DSA. After the exclusion of 5 patients who were CTA lost to follow-up, a total of 47 patients were included in this study.
Data collection and processing
The patient demographic data were obtained from the medical records of Maharaj Nakorn Chiang Mai Hospital (Digicard computerised system) from January 2013 to July 2022 using the term non-traumatic subarachnoid haemorrhage (ICD-10, code I60.9). Data collected from medical records included gender, age, risk factors, specifically family history of cerebral aneurysm and smoking behaviour, presenting symptoms, the Glasgow Coma Scale (GCS), clinical severity as defined by the World Federation of Neurosurgical Societies (WFNS), length of hospital stay, interval between the onset and repeat CTA, clinical symptoms of suspected recurrent SAH or re-ruptured vascular lesion, follow-up years, and the modified Rankin scale (mRS). Once pertinent patients were identified, images from the Picture Archiving and Communication System (PACS) of the Division of Diagnostic Radiology were reviewed.
The imaging data were collected by reviewing unenhanced CT brain, CTA brain, and cerebral DSA, as detailed below.
Unenhanced CT brain
Pattern of SAHs:
perimesencephalic pattern – SAH is classified as blood located interpeduncular, prepontine, premedullary, ambient, and/or quadrigeminal plate cisterns with no extension to the ventricular system [6];
diffuse pattern – blood centres in suprasellar or central basal cisterns and extends peripherally into the Sylvian fissure or cortical sulci.
Neurovascular imaging (CTA and DSA)
By consensus, interventional neuroradiologists and dia-gnostic radiologists reviewed all images. Images of each patient were interpreted at an interval of at least 2 weeks between reading sessions with randomisation and blindness to clinical information. All images were reviewed digi-tally at a workstation with the same large, high-resolution monitor and the same optimal window setting.
Imaging protocol:
Non-invasive imaging was performed with multidetector CT scanners (SOMATOM force; Siemens-Healthineers, Germany, 384 (2 × 192) slices) by using a 5-mm and 1.5-mm-thick axial section with a 3-mm-thick coronal or sagittal reconstruction of unenhanced CT. CTA coverage was from skull base to vertex, the following parameters being used: 120 kVp, adjusted mAs, collimation 64 × 0.625 mm, matrix 512 × 512, pitch 1.015 section, thickness 2 mm (section distance 1.6 mm) and 5 mm (section distance 5 mm) with axial, coronal, and sagittal MIP, VRT range, and MIP range. A total of 35 ml of nonionic contrast medium (Omnipaque 350; GE Healthcare) was intravenously administered at a rate of 3-4 ml/s via an automatic injector and then flushed with NSS 35-50 ml followed by application of the bolus tracking technique (ROI at carotid artery about C4-C5 level about 100 HU).
Cerebral DSA was carried out by 2 interventional neuro-radiologists using the same biplane vascular imaging system (Infinix VF-i/BP; Toshiba Medical Systems). In summary the following was done:
– DSA protocol: selective catheterisation and contrast injection of bilateral internal carotid arteries and at least one vertebral artery. In cases where reflux of contrast to the contralateral vertebral artery and the posteroinferior cerebellar artery was not achieved from a vertebral artery injection, we catheterised the contralateral vertebral artery injection. Standard 2D DSA in anteroposterior (AP) and lateral views and rotational 3D-DSA with maximum intensity projection (MIP) 3 views in 1-mm thickness were routine imaging protocols in our institution.
– The procedure was performed either under local or general anaesthesia with a right femoral artery approach using the same simple or complex diagnostic catheter.
– The flow rate of contrast media (Optiray 300: Ioversol 300 mgI/ml) was injected simultaneously into ICA at rates of 4 ml/s, volume 8 ml, and vertebral artery rates of 5 ml/s, volume 12 ml.
Clinical follow-up
After they were discharged, all the patients were closely watched for any signs of a recurrent SAH or re-ruptured aneurysm, guide symptoms including severe headaches, nausea and vomiting, blurred vision, sudden changes in consciousness, or neurological deficits. The clinical follow-up was every 3 months in the first year and every 6 months thereafter. Radiologic evaluation by CT and CTA was performed immediately if clinically indicated.
Statistical analysis
The qualitative data were expressed as numbers and percentages. The quantitative data were displayed as mean and SD. The statistical differences in demographic data and follow-up clinical outcomes were compared between the 2 study groups using Fisher’s exact test, the c2 test, and the Mann-Whitney U test. We evaluated the diagnostic yield of delayed CTA using percentages. The data analysis was performed using STATA software 16.1. A significant difference was considered at p < 0.05.
Results
Out of 422 patients who presented with non-traumatic SAHs, 52 individuals (12.3%) who were initially identified as having a negative DSA finding were enrolled onto our study. Following an initial negative DSA, all patients underwent standard follow-up CTAs within one to 2 weeks, in accordance with the protocol of our institution. After exclusion of 5 patients with no follow-up CTA, the final sample size for this study was 47 patients.
The demographic, clinical, and radiological parame-ters of the patients with spontaneous SAH, negative initial CTA, and DSA are summarised in Table 1. Of the 47 patients, the median age was 55 years (range: 28-81 years); 33 patients (70.2%) were male and 14 (29.8%) were female. The most common underlying disease was hypertension, accounting for 15 patients (31.9%). The most common symptom experienced was headache (70.2%), followed by collapse or loss of consciousness (19.1%), seizure (4.8%), confusion (2.1%), vertigo (2.1%), and weakness (2.1%). Only 10 patients (21.3%) were smokers. None of the patients had a family history of cerebral aneurysms.
Table 1
Comparison of demographic, clinical, and radiological parameters between perimesencephalic and diffuse SAH patients
Patients were divided into 2 subgroups based on blood distribution when presenting with an unenhanced CT brain scan: 16 patients (34%) in the perimesencephalic-SAH group and 31 patients (66%) in the diffuse-SAH group. There were no significant differences between these 2 groups regarding age, gender, underlying disease, risk factors, including smoking, and family history of cerebral aneurysm.
The World Federation of Neurosurgical Societies (WFNS) subarachnoid haemorrhage grading system was used to determine the clinical severity and prediction of survival rate. The perimesencephalic-SAH group scored a good grade of 100%, which is statistically significantly better than the diffuse-SAH group. Patients with diffuse SAH had a good WFNS grade in 58.1% of cases. The mean hospital stays were shorter in the perimesencephalic-SAH group (11.4 days) in comparison to the diffuse-SAH group (19.5 days).
The clinical outcome was obtained at least 6 months after discharge by using the modified Rankin scale, classifying “favourable outcome” as mRS scale 0-2 and “unfavourable outcome” as mRS scale 3-5. All members of the perimesencephalic-SAH group had favourable clinical outcomes. An unfavourable clinical outcome was only detected in 5 patients (16.1%), all from the diffuse-SAH group.
The repeated CTA following the negative initial DSA was performed on all patients. The mean interval between clinical presentation and repeat CTA was 24 days in both groups. This repeated CTA demonstrated 2 causative vascular lesions for all SAHs (overall yield of 4.3%), a yield of 0% in the perimesencephalic-SAH group, and a yield of 6.5% in the diffuse-SAH group (Table 2). The causative vascular lesions were specifically a small mycotic aneurysm (Figure 1) and a right PICA aneurysm (Figure 2). The aneurysm in case 1 was treated with antibiotics, and in the follow-up CTA there was no evidence of injury, and the aneurysm in the second case underwent aneurysm trapping.
Table 2
Results of repeat CTA after negative initial DSA in perimesencephalic and diffuse SAH
| Parameter | Total (n = 47) | Perimesencephalic SAH (n = 16) | Diffuse SAH (n = 31) | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Repeat CTA, n (%) | |||||||||
| Positive | 2 (4.3) | 0 (0.0) | 2 (6.5) | 0.541 | |||||
| Negative | 45 (95.7) | 16 (100.0) | 29 (93.5) | ||||||
Figure 1
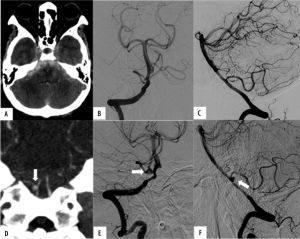
A 70-year-old woman with infective endocarditis presents with a thunderclap headache. A) Unenhanced computed tomography demonstrates a diffuse pattern of subarachnoid hemorrhage with mild hydrocephalus. B) Frontal view of right internal carotid artery (ICA) injection of initial digital subtraction angiography (DSA) showed a focal irregularity of the temporal branch of right middle cerebral artery (MCA) by retrospective review (arrow). C) A repeated computed tomography angiography (CTA) performed 11 days after the initial DSA demonstrated interval growth of the mycotic aneurysm at the temporal branch of the right MCA (arrow). This patient underwent antibiotic treatment
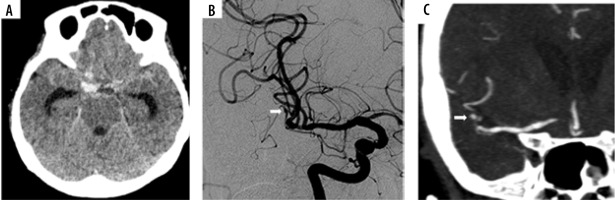
Figure 2
A 62-year-old woman presents with a severe headache. A) Unenhanced computed tomography demonstrates a diffuse pattern of spontaneous subarachnoid haemorrhage (SAH), predominant in the posterior fossa. B, C) Initial digital subtraction angiography (DSA) of the right vertebral artery injection in anteroposterior (AP) and lateral views does not demonstrate a vascular abnormality. D) Follow-up computed tomography angiography (CTA) performed 9 days after DSA demonstrated a small aneurysm at the right PICA origin (arrow). E, F) Secondary DSA in AP and lateral views confirmed a right PICA aneurysm (arrow). This patient underwent a suboccipital craniotomy (SOC) to trap an aneurysm
The average follow-up period for clinical symptoms was 4.7 years. No evidence of rebleeding SAH was found in any case. However, there were 2 patients in the diffuse-SAH group who developed clinical headache during follow-up, but there was no evidence of SAH or re-ruptured aneurysm from the unenhanced CT and CTA.
Discussion
Idiopathic SAH, which affects 10 to 30% of all SAH patients [11, 13], is identified from negative DSA, which is carried out to detect any bleeding cause. According to our results, the incidence of SAH that is DSA negative is likely to be 12.3% according to the range recorded in rele-vant literature. Undiagnosed aneurysmal SAHs or other vascular malformations carry a risk of rebleeding, which can increase mortality and morbidity. Therefore, repeated neurovascular imaging has received support from nume-rous authors [14-18].
In the routine protocol at our centre, we used CTA to follow SAH patients whose DSA findings were negative. This was because CTA has a high specificity and sensitivity of 98% for detection of aneurysms [19], is noninvasive, and has a very low risk of permanent neurological deficit. The overall yield of our study, which considers the yield of repeat CTA in patients with SAH and a negative initial DSA exam, is 4.3%. This corresponds to the results of other studies, assessing the 3.7 to 11.4% yield of repeat CTA [6, 11, 12].
Perimesencephalic subarachnoid haemorrhage appears to have a different aetiology and natural history than aneurysm rupture, with favourable clinical outcomes and no evident source of bleeding. The aetiology is believed to be a deep venous haemorrhage [20]. This is referred to as non-aneurysmal perimesencephalic SAH. According to our study, patients in the perimesencephalic SAH group had a comparatively benign clinical course, including a reassuring result from the WFNS-SAH grading system (100%), a shorter hospital stay (mean 11.4 days), and favourable clinical outcomes (mRS 0-2 100%) superior to diffuse SAH. All our patients with perimesencephalic SAH had negative DSA and follow-up CTA results, and all follow-up CTAs in these patients had a diagnostic yield of 0%, which corresponds to the findings described by Sadigh et al. [11] and Almandoz et al. [6]. A meta-analysis of 40 studies [10] investigated a total of 1031 patients with perimesencephalic SAH. Only 0.78% were diagnosed during follow-up DSA. According to the results, repeated imaging with CTA is optional or preferred in individuals whose clinical presentation does not correspond to that of perimesencephalic SAH or who have atypical imaging abnormalities such as vasospasm and hydrocephalus. We believe that the second DSA in the perimesencephalic SAH does not warrant repetition, which is in accordance with the results of the Yeole et al. trial [7]. Diffuse SAH requires more careful consideration regarding an identifiable cause of haemorrhage than perimesencephalic SAH, particularly in the case of ruptured aneurysms. Out of the 31 patients diagnosed with diffuse SAH in our study, we found that 2 cases had positive follow-up CTAs from occult ruptured aneurysms, resulting in a yield of 6.5%. Previous studies conducted by Sadigh et al. [11] and Almandoz et al. [6] reported yields of 7.7% and 8%, respectively, in the diffuse SAH group.
The cause of diffuse SAH in the first case (Figure 1) detected by repeat CTA in our study was a mycotic aneurysm at the anterior temporal branch of the right middle cerebral artery (MCA), which was well visualised in the follow-up CTA after its interval growth. In retrospective assessment of the initial DSA, it presented with a focal peripheral irregular lesion, which was, however, an inconclusive finding, and too small to diagnose a cerebral aneurysm. Closed follow-up imaging is helpful in the case of a small, inconclusive lesion.
In the second case of diffuse SAH (Figure 2), initial DSA was unable to detect any potential vascular abnormalities. A follow-up CTA was performed 9 days after the initial DSA and revealed interval growth of the small aneurysm at the right PICA origin.
As yet, there is no established international standard for imaging follow-up in initial DSA-negative SAH. Repeat DSA has been advocated for occult aneurysm detection in numerous trials [7, 14-18, 21-29]; however, this invasive process entails additional neurological complications and costs for care, whereas the current multidetector CTA study provides a fast, simple, and noninvasive diagnostic method. Recently, Wu et al. [12] have suggested that the specificity, overall sensitivity, and accuracy of 320-row CTA are comparable to those of DSA for the rate of cerebral aneurysm detection; as a result, this method may take the position of DSA as the primary choice for noninvasive assessment of follow-up initial DSA-negative SAH patients.
In this research, we followed up on initial DSA-negative SAH using modern advanced CT 384 (2 × 192) slices. The follow-up CTA was also warranted and performed effectively because it uncovered 2 undetected vascular lesions for which the initial DSA had yielded negative or inconclusive results. It is significant that no patients experienced rebleeding throughout the monitoring period for at least 2 years (mean 4.7 years). Following the protocol at our institution, we think that for patients with non-traumatic SAH, looking at 4 vessels with 3D rotational DSA during the first evaluation and then a modern advanced CT 384 (2 × 192) slices within 1-2 weeks may lower the chance of missing the vascular lesions.
The limitation of this study was its retrospective methodology, small patient population and study length. A large, prospective, and multicentre trial is needed to confirm these results.