Introduction
The prevalence of primary hypertension (PH) in the child and adolescent population is in the phase of continuous growth [1]. Lack of physical activity, sedentary lifestyle, and a diet based on highly processed products (associated with a progressive “plague” of obesity) among young patients is an issue that has been accumulating for several years [2], now additionally accelerated by the COVID-19 pandemic, which significantly impeded access to physical activities.
Arterial hypertension is associated with arterial wall remodeling, which leads to a decrease in its flexibility. This process lowers the compliance of the arteries (including the aorta, which may contribute to the occurrence of primary coronary events) and increases the pulse wave velocity (PWV) in proportion to the severity of hypertension [3]. It is caused by several changes – an increase in thickness of the aortic wall and large arteries, and increases in the cross-sectional area and the diameter of the lumen of these vessels [4]. This remodeling is reversible at an early stage. Therefore, effective and early therapy of hypertension may cause the changes in the structure of the arteries to regress. Thus, reducing systolic and diastolic blood pressure and pulse pressure, and arterial stiffness, is one of the most critical goals in PH therapy [5].
Furthermore, the assessment of pressure in elastic arteries, i.e., central blood pressure (CBP), seems to be the most appropriate method of assessing the severity of changes in progressive PH, as well as the improvement of the changes and the correctness of the implemented therapy in relation to the standard measurements of the peripheral office blood pressure (BP) [6, 7].
Single pediatric studies highlight the importance of measuring central pressure in children. Central blood pressure was higher in children with autosomal dominant polycystic kidney disease [8], and CBP strongly correlated with left ventricular mass in children with primary hypertension [9]. Also, 24-hour (24-h) blood pressure monitoring (ABPM) is an important, widely used tool in pediatrics [10], clearly better correlating with organ changes than office measurement [11]. Many studies have demonstrated its usefulness, such as the ESCAPE (The Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients) study [12]. Scientific societies recommend routine ABPM testing in children at risk, such as patients with chronic kidney disease (CKD) [13]. Considering the advantages of these two methods, the recently introduced 24-h central ABPM seems to be the ideal method to assess not only blood pressure but also the cardiovascular phenotype of the patient.
In the last two decades, the role of immune system activation in the pathogenesis of PH has been extensively studied [14]. Undoubtedly, primary hypertension is considered a state of low-grade inflammation [7] nowadays. The extent of subclinical inflammation can be measured using, e.g., the concentration of proinflammatory cytokines but also with simple complete blood count (CBC)-derived markers such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and mean platelet volume (MPV). In adults, elevated NLR, PLR, and MPV were found in hypertensive patients and were proposed as markers of increased risk for mortality [15, 16]. In our previous studies, we have seen elevated NLR and PLR in PH patients compared to healthy children and their relation to hypertension-mediated organ damage (HMOD): arterial stiffness and microalbuminuria [17, 18].
This study aimed to evaluate the usefulness of 24-h CBP measurement in adolescents with PH and analyze the relation between 24-h CBP and immune system activation in this group of patients.
Material and methods
In our single-center cross-sectional study, we analyzed 28 patients aged from 10.75 to 17.92 years with PH treated in one tertiary center of pediatric nephrology. A confirmed diagnosis of primary hypertension was the inclusion criterion [19]. Exclusion criteria were secondary hypertension, any pharmacological antihypertensive treatment administered, any diagnosed disease of the kidneys or circulatory system, chronic inflammatory disease, and symptoms of acute infection. The control group consisted of 25 age- and sex-matched healthy, normotensive children.
Approval from the local Bioethical Committee was obtained before initiating the research (approval no. KB/58/2016, 15 March 2016). All procedures involving human participants were in accordance with the highest ethical standards of the institutional research committee and with the Declaration of Helsinki on the treatment of human subjects and its later amendments. Informed consent was obtained from all participants (≥ 16 years) and their representatives included in the study.
For every patient upon admission, we established basic anthropometric features: height [cm], weight [kg], BMI [kg/m2], peripheral systolic blood pressure (SBP) [mmHg], and peripheral diastolic blood pressure (DBP) [mmHg] measured using an oscillometric method and Welch Allyn Vital Signs Monitor 300 (Welch Allyn Inc., Skaneateles Falls, NY, USA). Those measured values were compared with normative data and expressed as Z-scores [20, 21].
We examined blood samples taken from every patient assessing the following parameters using routine laboratory techniques: number of neutrophils (NEU) [1000/µl], lymphocytes (LYM) [1000/µl] along with complete blood count-derived inflammatory markers: MPV [fl], NLR, and PLR. In serum, we measured concentration of uric acid [mg/dl], total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol [mg/dl], and triglycerides [mg/dl]. Glomerular filtration rate was estimated according to the revised Schwartz formula (eGFR) [ml/min/1.73 m2] [22].
We performed ambulatory blood pressure monitoring using Oscar 2 Suntech with Sphygmocor inside (AtCor Medical Pty Ltd., Sydney, Australia). The following basic peripheral blood pressure parameters were analyzed: peripheral systolic, diastolic, and mean blood pressure during 24 hours (SBP, DBP, MAP) [mm Hg], MAP 24-h Z-score, blood pressure loads [%], and nocturnal peripheral systolic and diastolic blood pressure dip (DIP SBP, DBP) [%] [23]. The most significant attribute of Oscar 2 Suntech with Sphygmocor inside is its ability to evaluate values for CBP using oscillometric technique and the transfer function known from the Sphygmocor device. By this means, we assessed the following parameters during 24 hours: central systolic, diastolic, and mean blood pressure (cSBP, cDBP, cMAP), central augmentation pressure (cAP) [mmHg], central augmentation index (cAIx) [%] and augmentation index corrected for a heart rate of 75 beats per minute (cAIx75HR) [%] and ambulatory arterial stiffness index (AASI) calculated automatically by ABPM software according to Li et al. [24]. Amplification of SBP was defined as the difference between peripheral and central systolic blood pressure [25].
All results were analyzed using Dell Statistica 13.0 (TIBCO Soft., Aliso Viejo, Ca, USA), and all the data were presented as mean values and standard deviations. The normality of data distribution was checked using the Shapiro-Wilk test. Continuous variables were compared with Student’s t-test or the Mann-Whitney U-test. A comparison of categorical variables was performed with the χ2 test. Pearson’s linear correlation coefficient and Spearman’s rank correlation coefficient were determined to investigate associations between quantitative variables. A two-sided p-value less than 0.05 was considered statistically significant.
Results
A complete summary of the measured clinical and biochemical parameters is presented in Table 1. Out of the entire group of measured clinical and laboratory parameters, compared with the study and control groups, only BMI Z-score, uric acid, and triglycerides serum concentrations were significantly different. There was no significant difference in markers of subclinical inflammation – NLR, PLR, MPV – between PH and healthy children.
Table 1
Clinical and biochemical parameters in the study and control group
The performed measurements showed many differences in the values of peripheral arterial pressure between PH and normotensive peers (Table 2). Both systolic and diastolic office and ABPM pressures were significantly higher in the group of patients diagnosed with primary hypertension. Additionally, in the case of 24-h ambulatory measurements, a statistically significant difference was noted for mean arterial pressure, pulse pressure, and systolic and diastolic pressure loads. All of these parameters were higher in the group of patients with diagnosed primary arterial hypertension.
Table 2
Peripheral blood pressure
Considering the parameters characterizing central arterial blood pressure and stiffness of large arteries separately, we found a statistically significant difference for all CBP values and none of the indirect indicators of increased arterial stiffness. All of these parameters are presented in Table 3.
Table 3
24-h ambulatory central blood pressure and parameters of arterial stiffness
[i] ABPM – ambulatory blood pressure monitoring, cSBP – central systolic blood pressure, cDBP – central diastolic blood pressure, cMAP – central mean blood pressure, cPP – central pulse pressure, cAP – central augmentation pressure, cAIx – central augmentation index, cAIx75HR – central augmentation index corrected for heart rate of 75 beats per minute, DIP – blood pressure dipping, AASI – ambulatory arterial stiffness index
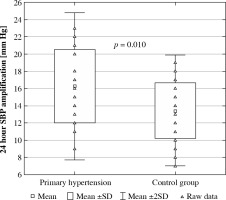
Peripheral and central blood pressure values in both groups are presented in Table 4. In both groups, 24-hour central systolic blood pressure and 24-h central pulse pressure were significantly higher (p < 0.001) compared to peripheral systolic blood pressure and peripheral pulse pressure, respectively. There were no differences in central and peripheral diastolic and mean blood pressure in normotensive and hypertensive subjects. The amplification of systolic blood pressure was significantly higher in PH patients compared to healthy peers (16.25 ±4.27 vs. 13.44 ±3.23 mmHg, p = 0.010) (Fig. 1).
Table 4
Comparison of central and peripheral blood pressures in the study and control group
Fig. 1
Amplification of central systolic blood pressure (SBP) in patients with primary hypertension and healthy peers
Correlations of peripheral and central blood pressure, and stiffness indices with clinical and biochemical parameters in 53 patients are presented in Table 5. BMI Z-score correlated with both peripheral and central systolic blood pressure, central mean blood pressure, central and peripheral pulse pressure, and stiffness indices. Uric acid serum concentration correlated with central and peripheral systolic and pulse pressure, systolic blood pressure amplification, and arterial stiffness measured as AASI.
Table 5
Correlations of peripheral and central blood pressure, and stiffness indices with clinical and biochemical indices in the whole group of patients
[i] SBP – systolic blood pressure, ABPM – ambulatory blood pressure, BMI – bodymass index, c – central blood pressure, MAP – mean arterial pressure, PP – pulse pressure, SBPL – systolic blood pressure load, cAP – central augmentation pressure, cAIx – central augmentation index, AIx75HR – central augmentation index corrected for heart rate of 75 beats per minute, AASI – am-bulatory arterial stiffness index
Table 6 presents correlations of peripheral and central blood pressure with inflammatory indices. In the whole group of 53 patients, 24-h peripheral and central systolic, diastolic, and mean blood pressure correlated positively with the neutrophil count, neutrophil-to-lymphocyte, and platelet-to-lymphocyte ratios. Central stiffness indices correlated only with platelet count. The latter parameter also correlated with central mean blood pressure. Otherwise, mean platelet volume correlated only with peripheral diastolic blood pressure.
Table 6
Correlations of peripheral and central blood pressure and stiffness indices with inflammatory indices in the whole group of patients
[i] SBP – systolic blood pressure, ABPM – ambulatory blood pressure, c – central blood pressure, BMI – body mass index, DBP – diastolic blood pressure, MAP – mean arterial pressure, L – blood pressure load, cAP – central augmentation pressure, cAIx – central augmentation index, AIx75HR – central augmentation index corrected for heart rate of 75 beats per minute, NLR – neutrophil-to-lymphocyte ratio, PLR – platelet-to-lymphocyte ratio, MPV – mean platelet volume
In addition, in the group of 28 adolescents diagnosed with primary hypertension, parameters of arterial stiffness were associated positively with BMI Z-score (cAP: R = 0.415, p = 0.028, AASI: R = 0.487, p = 0.010), LDL (cAP: R = 0.430, p = 0.028, cAIx: R = 0.471, p = 0.015, cAIx75HR: R = 0.442, p = 0.024) and uric acid (AASI: R = 0.430, p = 0.028) and negatively with HDL (AASI: R = –0.428, p = 0.033). Central but not peripheral pulse pressure correlated with HDL cholesterol (R = –0.407, p = 0.039).
In the whole group of patients but also in both subgroups there was a very strong positive correlation between 24-h ABPM peripheral blood pressure and the corresponding CBP (for whole group of patients – SBP vs. cSBP: R = 0.927, p < 0.001; DBP vs. cDBP: R = 0.987, p < 0.001; MAP vs. cMAP: R = 0.925, p < 0.001).
None of the analyzed inflammatory markers (NLR, PLR, MPV) correlated significantly with age or height.
Discussion
This is one of the first studies in the pediatric population analyzing 24-h CBP and the first study to analyze its relationship with the degree of subclinical inflammation. We found that central 24-h BP assessed using the oscillometric method is higher in patients with primary hypertension than in healthy peers, alongside peripheral blood pressure. The amplification of systolic blood pressure was significantly higher in hypertensive patients. Of note, 24-h CBP correlated positively with markers of low-grade inflammation, i.e., neutrophil count, neutrophil-to-lymphocyte, and platelet-to-lymphocyte ratios, as well as with mean platelet volume. It is worth emphasizing that we found numerous determinants of aortic stiffness in the group of adolescents with primary hypertension – increased platelet count, increased uric acid and LDL cholesterol, and decreased HDL cholesterol.
In many previous studies using both direct and indirect methods of measuring CBP, there was a repeated observation of the difference between the pressure measured at the peripheral artery and the CBP [6, 8, 9]. Central arterial pressure, i.e., measured in the aorta, was lower than the peripheral arterial pressure [6, 26]. The reason for this is most likely the difference in the structure of the wall of the arteries depending on their caliber. The larger vessels closer to the heart are mostly elastic, while the smaller peripheral vessels lose elastic fibers to the muscle layer. Moreover, the further the arterial vessel is located from the heart, the more its lumen and wall thickness decrease, although not at the same rate, i.e., the surface area of the lumen decreases faster than the thickness of the vessel wall. Thus, the smaller the vessel is, the lower is the ratio of the vessel lumen to the cross-sectional area of the vessel as a whole. Both of these factors – the change of the structure from flexible to muscular and the decrease in the ratio of the lumen area to the cross-sectional area – result in the growth of pulse wave velocity and the amplification of peripheral blood pressure.
Interestingly, the phenomenon of amplification was statistically significant only in the case of SBP. The fact that there was no similar dependence for diastolic or mean blood pressure agrees with reports from the previous studies [6]. Isolated systolic hypertension (ISH) is a prevalent form of arterial hypertension among children, adolescents, and young adults. There are many hypotheses for the pathogenesis of ISH in young patients. One states that ISH in these individuals is related to high aortic elasticity and high peripheral amplification, leading to normal central and abnormally elevated peripheral SBP [25]. In line with this hypothesis, our PH patients were characterized by significantly higher SBP amplification than healthy peers.
Our study revealed an association between CBP and excess body mass, expressed in the form of a BMI Z-score. It was not the aim of this study to investigate how overweight and obesity affect the development of arterial hypertension. Still, the fact is that in the children and adolescents suffering from primary hypertension, the mean BMI was significantly higher. This observation is in line with the results of our previous studies [17, 18]. It may confirm that children maintaining the correct weight for age, height, and sex develop arterial hypertension less frequently [27].
The only biochemical parameter significantly related to hypertension was uric acid serum concentration. A link between hyperuricemia and the risk of hypertension has been revealed in both experimental and clinical studies [28, 29]. In addition, even a treatment program for hypertensive adolescents was established; it included a xanthine oxidase inhibitor (allopurinol) or a uricosuric agent (probenecid), which are used to reduce uric acid concentration in blood [30, 31].
Numerous studies have found a link between the development of primary hypertension, HMOD, and subclinical inflammation in both adults [32] and children [33]. Researchers have already described several pathophysiological mechanisms over the past few years. They formulated hypotheses that link the role of sodium ions with immune system activation. These ions are supposed to fulfill a dual role – firstly as a factor in the development of hypertension and secondly as a proinflammatory factor, increasing the production of inflammatory cytokines and reactive oxygen species and weakening the activation of anti-inflammatory mechanisms by inhibiting macrophages and regulatory T-lymphocytes. Reactive oxygen species and proinflammatory cytokines can raise blood pressure through increased sodium retention and vasoconstriction. These negative phenomena, along with the activated renin-angiotensin system, can damage the endothelium and lead to arterial stiffness and decreased vascular flow [32, 33]. The exacerbation of subclinical inflammation can be assessed by determining the concentration of proinflammatory cytokines (e.g., interleukin 18 or high-sensitivity C-reactive protein), but also indicators that are much simpler to determine such as NLR, PLR, and MPV, which were found to be associated with poor prognosis for several cardiovascular diseases in studies performed in groups of adult patients [16, 34, 35].
In our study, we observed positive correlations between inflammatory markers, such as NLR and PLR, and the average 24-h central pressure – cSBP, cDBP, cMAP, cPP – in all the children. To the best of our knowledge this is the first study to reveal the relation between 24-h CBP and immune system activation in this group of patients.
However, it should be noted that the relationships with inflammation observed for CBP are not significantly different from those also found for peripheral blood pressure. We have found only a few individual relationships unique to CBP. In the oscillometric method using a single cuff (as in the Oscar 2 with Sphygmocor Inside used in this study), central pressure is a mathematical function of peripheral pressure. Very high correlations between both types of pressures were found (R > 0.900). On the other hand, CBP seems to be more relevant to damage to the brain, heart, and kidneys and future events. Furthermore, measurement of the central waveform provides clinically useful information, such as the quantification of wave reflections with the augmentation index, beyond blood pressure measured in the brachial artery [36]. Further studies are needed to assess the utility of this new promising technology as a predictor of outcome and HMOD in pediatric patients with PH.
Our results are in line with the study of Gackowska et al. [37]. The authors analyzed office CBP (not 24-h central ABPM) in relation to lymphocyte receptor expression (CD – a cluster of differentiation) determined using flow cytometry. Central blood pressure correlated with the proportion of CD31 deficient T lymphocytes. As the CD31-bearing T-cell population represents a cellular marker for thymic activity, the authors concluded that pediatric patients with PH are characterized by decreased thymic function [37]. Premature immune system maturation may be one of the features of PH alongside other elements of accelerated development, including early vascular aging and the early growth spurt [38].
Many studies have confirmed the role of parameters characterizing arterial stiffness as markers of progression of the vascular remodeling process in the course of arterial hypertension and thus as markers of progression of the disease itself [26]. As a standard, arterial stiffness is assessed by direct methods, using ultrasound to test the thickness of individual layers of the arterial wall, its elasticity, compliance, and using oscillometry or applanation tonometry to evaluate pulse wave velocity and shape, which better illustrate the progression of the vessel remodeling process [39]. In our study, we used an indirect method that uses only the transfer function to analyze the pulse wave measured in the peripheral artery to calculate arterial stiffness parameters. Notably, this method and device have not been validated in adult and pediatric populations yet. This fact, along with the relatively small size of the studied groups, could contribute to the lack of significant differences in the values of the obtained parameters of arterial stiffness between the study and the control groups.
Despite the limitations of the method used, we found a positive correlation between arterial stiffness indices and platelet count, which is in accordance with the results of our previous study [18] and suggests that evaluation of a CBC-derived inflammatory marker may serve as a preliminary marker of arterial damage in pediatric patients with PH. Interestingly, our results are consistent with a recently published Chinese adult study that found a positive relation between platelet count and arterial stiffness [40]. It is not clear yet how dysregulation of the number and function of platelets might contribute to the development of hypertension-mediated arterial damage. Increased platelets are associated with elevated levels of plasminogen activator inhibitor type 1 (PAI-1), which is involved in the development and progression of atherosclerosis [41].
Our study confirmed well-known relations between BMI, lipid disturbances, uric acid, and arterial damage. Two interesting observations from our research are the positive correlation between 24-h AIx75HR and LDL level and the negative correlation between AASI and HDL level revealed in the study group. It may lead to the conclusion that dyslipidemia is an additional factor stimulating the process of vascular remodeling in children with PH. Similar relations were already found for direct methods of measurement of arterial stiffness in both children [42] and adults [43].
Some limitations to the study should be mentioned. Firstly, the cross-sectional nature of our study precludes drawing final conclusions on the mutual relation between CBP and the analyzed parameters. Secondly, the small number of examined subjects could have influenced the results – i.e., no difference in the stiffness parameters or NLR and PLR between the groups. Also, the device used in the study has not been validated yet to evaluate arterial damage against gold standard methods. In addition, we analyzed only CBC-derived parameters of low-grade inflammation, not more precise indicators of immune activation, e.g., interleukins, high-sensitivity C-reactive protein (hsCRP), or lymphocyte surface antigens such as clusters of differentiation (CD). On the other hand, we have previously demonstrated the usefulness of these parameters in our other studies on PH and CKD patients [17, 18].
Conclusions
Increased central 24-h blood pressure and systolic blood pressure amplification may be associated with immune system activation in adolescents with primary hypertension.
In adolescents with primary hypertension, dyslipidemia and hyperuricemia are risk factors for increased arterial stiffness.
Further studies on central and peripheral blood pressure in terms of their relationship with inflammation in these patients are needed.


