Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) is one of the most commonly used diagnostic and therapeutic interventional modalities for pancreaticobiliary diseases (Photo 1). However, recent trends over the last two decades have reduced its employment as a diagnostic procedure, while non-invasive imaging techniques have become more popular as a diagnostic tool [1]. The most common use and indication for ERCP is the removal of stones from the biliary tree; other indications are malignant, inflammatory or postoperative strictures, ampullary masses causing obstruction, sphincter of Oddi dysfunction, bile leaks due to gallbladder and liver surgery, cholecystitis, cholangitis, acute, recurrent or chronic pancreatitis, pancreas divisum, pancreatic duct leaks, fluid collections, drainage of cystic lesions of pancreas, and stenting of pancreatic tumors. The procedures are sphincterotomy, dilatation and removal of stones, drainage and stenting, cholangiography biopsy and manometry [1, 2].
Although ERCP is considered to be a safe procedure, it may have significant complications such as cholangitis (1–1.4%), hemorrhage (2%), pancreatitis (1–15.7%), perforation (< 1%), and strictures developing over months to years [3]. The incidence of post-ERCP pancreatitis (PEP) widely varies according to studies and definitions, as much as between 1% and 15.7% [3, 4]. Serum pancreatic enzymes are elevated because of minor trauma to the pancreatic parenchyma imputable to manipulations during ERCP; however, asymptomatic hyperamylasemia is a more frequent situation that should be distinguished from real acute PEP and is reported between 6.8% and 70% [5].
ERCP is performed on an outpatient basis in most hospitals (Photo 2). The first control of amylase and hemogram is noted at 2 or 4 h after ERCP. LaFerla et al. [6] were early to identify that serum amylase levels rose quickly 2 h after ERCP. PEP is defined as pancreatic type abdominal pain with increased levels of serum amylase more than 3 times the upper normal limit at 24 h after ERCP [7]. To standardize the diagnosis, revised Atlanta Criteria have been introduced which take imaging and organ failure into consideration [8]. Furthermore, performing a physical examination at 2 or 4 h after ERCP is controversial due to the effect of anesthesia and the distention of the abdomen due to air insufflation, as well as other causes such as perforation [9]. Pancreatitis may result in pancreatic infection-sepsis, necrosis, multi-organ failure and eventually death. Once a patient is diagnosed with PEP, every effort should be made to prevent complications of pancreatitis.
Aim
The aim of this study is to identify the laboratory markers and patient characteristics that may be used to predict or to define the difference between transient hyperamylasemia (TH) and real acute pancreatitis.
Material and methods
Study design and patient selection
Medical files of patients with choledocholithiasis who underwent ERCP were evaluated in a retrospective cohort study at the General Surgery Department between November 2015 and October 2018. Local ethics committee approval was granted by the number of 528/21.06.2018, with full confirmation of the Helsinki Declaration. Inclusion criteria were as follows: all emergency and elective choledocholithiasis cases were included if the patients had their first ERCP. Only successfully completed ERCPs were taken into account. Emergency cases were the patients with choledocholithiasis related cholangitis or mechanical icterus leading to deterioration of liver functions. Elective cases were the patients with choledocholithiasis with no biochemical abnormality; for these patients ERCP was scheduled within 2–3 weeks. Biliary pancreatitis cases were excluded.
Data collection
The patients were followed up for at least 24 h by checking amylase, white blood cell (WBC) count, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gammaglutamyl transferase (GGT), and bilirubin levels at 6 and 12 h after ERCP with physical examinations. If laboratory abnormalities with pancreatic type abdominal pain were detected at these observational timepoints, the patients were checked again at 24 h; otherwise, they were discharged. The patients with other complications such as perforation, bleeding with or without TH, and PEP were excluded to maintain homogeneity and easy assessment of hyperamylasemia in the study group. Hence, 75 patients with PEP or TH and 168 normal patients in a total of 243 patients constituted our study population.
The interventional procedure
The ERCP procedures were performed by two surgical interventional gastrointestinal endoscopists with experience of more than 1000 thousand upper and lower tract gastrointestinal endoscopies and more than 100–120 ERCPs annually in the last 5 years. Patients were hospitalized after at least 8 h fasting and the ERCP procedure was carried out in the operating room under sedation analgesia anesthesia. A sphincterotome was inserted into the papilla; if the endoscopist managed to advance the sphincterotome, controlled small amounts of contrast medium was given under fluoroscopy to reveal opacification of the common bile duct (CBD) or pancreatic duct (PD). In circumstances of pancreatic duct opacification, the sphincterotome was withdrawn and manipulated in the direction of the CBD. If cannulation of the CBD was evidenced with opacification, a 10 mm sphincterotomy was performed. If the technique failed, a guidewire was used to ease the cannulation, and a guidewire was also used at the failed first attempt of cannulation of the papilla with the sphincterotome. In the case of failed papilla cannulation, a precut sphincterotomy was performed by a needle knife papillotome. Following papillotomy, the sphincterotome with guidewire was again inserted into the CBD and sphincterotomy was completed. Balloon and basket catheters were inserted into the CBD for extraction of stones. This standardized procedure was performed in all patients. None of the patients were given perioperative medication for pancreatitis prevention and PD stents were not left placed.
Definitions
PEP and TH are different entities. PEP needs a lengthened hospitalization whereas a TH patient can be discharged.
The current PEP definition was made according to Cotton criteria and revised Atlanta classification [7, 8]. The general definition is persisting and worsening abdominal pain at least 24 h with an amylase level of more than 3 times the upper limit at the 24th h.
Difficult cannulation was accepted as the achievement of cannulation after 15 min from the entrance of duodenoscope due to a greater number of attempts, and need for precut sphincterotomy.
Statistical analysis
IBM SPSS for Windows version 21.0 (Armonk, NY: IBM Corp) was used for statistical analysis. Descriptive statistics were given as number and percentage for categorical variables, and mean and standard deviation for numerical variables. If the numerical variables were normally distributed, Student’s t-test was used to compare two independent groups. If the numerical variables were not normally distributed, the Mann-Whitney L test was used to compare two independent groups. The χ2 test was used for the rates of categorical variables among groups. Determinant factors were analyzed logistic regression analysis. The cut-off value was analyzed by ROC curve analysis. P-values less than 0.05 were considered statistically significant.
Results
The mean age of patients having post-ERCP hyperamylasemia was 58.8 ±13.3 (range: 24–80). Forty-eight (64%) patients were female and 27 (36%) were male. Forty-two (56%) patients had elective-planned ERCP and 33 (44%) patients had emergent ERCP, of whom 6 (8%) also had cholangitis. Sixty-nine (92%) patients had no cholangitis. Difficult ERCP was seen in 8 (10.7%) patients and Wirsung cannulation occurred in 12 (16%) patients. Forty-eight (64%) patients had no abdominal pain, while 15 (20%) patients had abdominal pain at the 12th h but no pain at the 24th h. Twelve (16%) patients had abdominal pain at the 12th and 24th h; these patients were evaluated as having PEP. The mean amylase levels were 468 (U/l) (190–852) at the 6th h, 364.3 (U/l) (94–748) at the 12th h and 799.3 (U/l) (517–1283) at the 24th h. The mean CRP levels were 16.9 mg/dl (4–138) at the 6th h, 21.5 mg/dl (5–140) at the 12th h and 98.3 (35–185) mg/dl at the 24th h. The mean WBC counts were 11.0 × 103 (1.5–21.2) at the 6th h, 10.2 × 103 (6–19.3) at the 12th h and 16.1 × 103 (10–21) at the 24th h (Table I).
Table I
Demographics and characteristics of the study group
For the development of PEP and differentiation from TH variables were compared and evaluated in Table II. The presence of cholangitis (p = 0.018), Wirsung cannulation (p = 0.008), presence of abdominal pain at the 12th and 24th h (p < 0.001), amylase level at the 12th h (p < 0.001), CRP levels at the 6th and 12th h (p = 0.001 and p < 0.001), and WBC levels at the 6th and 12th h (p = 0.001 and p < 0.001) were significantly higher for development of PEP. Amylase level decreased in patients who did not have PEP. CRP levels increased in both groups.
Table II
List of significant parameters related to post-ERCP pancreatitis
| Parameter | P-value |
|---|---|
| Sex | 0.655 |
| Emergent or elective | 0.648 |
| Cholangitis present | 0.018 |
| Difficult ERCP | 0.079 |
| Wirsung cannulation | 0.008* |
| Amylase at 6th h | 0.190 |
| Amylase at 12th h | 0.000* |
| Abdominal pain at 12th h | 0.000* |
| Abdominal pain at 24th h | 0.000* |
| CRP levels at 6th h | 0.001* |
| CRP levels at 12th h | 0.000* |
| WBC levels at 6th h | 0.001* |
| WBC levels at 12th h | 0.000* |
The most important factors for the development of PEP were Wirsung cannulation and the WBC count at the 6th h (Table II).
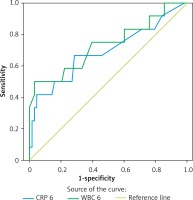
Receiver operating characteristic (ROC) curves were plotted for CRP to identify patients with PEP, with a statistically significant area under the curve of 0.690 (95% confidence interval (CI): 0.501–0.879) (Figure 1). We also calculated the positive predictive value (PPV), negative predictive value (NPV), positive likelihood (+LH) and negative likelihood (–LH) for CRP at a cut-off value of 8 mg/dl, which had a negative predictive value in approximately 70% of patients with a similar sensitivity (Table III).
Table III
Sensitivity, specificity, +LR and –LR, positive predictive value, negative predictive value of C-reactive protein levels for post-ERCP pancreatitis at optimal cut-off level
ROC curves were also plotted for WBC to identify patients with PEP, with a statistically significant area under the curve of 0.732 (95% CI: 0.552–0.912) (Table IV). We also calculated the PPV, NPV, +LH and –LH for RDW at a cut-off value of 10 × 103, which had a negative predictive value in approximately 90% of patients with a sensitivity over 80% (Table IV).
Table IV
Sensitivity, specificity, +LR and –LR, positive predictive value, negative predictive value of white blood cell count for post-ERCP pancreatitis at optimal cut-off level
Discussion
Several factors have been identified as a risk for development of PEP. Female sex has been found to be related to the development of PEP [10, 11]. This finding was not confirmed by our study model (p = 0.655). However, this finding is in agreement with the study by Katsinelos et al. [4].
Age has also been shown to be a significant factor in the development of PEP. Age younger than 60 years is found to be related to disease in several prospective studies; this finding was confirmed in our study. Overall, our patient population with hyperamylasemia was younger than 60 years of age [12, 13]. There was no significant difference in age between the PEP and transient hyperamylasemia groups (p = 0.281).
Urgent endoscopic biliary drainage was reported to be important in the treatment of acute cholangitis in a study by Boender et al. [14], in 1995, pointing out that adverse events due to persistent cholangitis became more frequent as the delay between onset and biliary drainage increased. This was later supported by Tan et al., in their large group study, which reported marginally significant improvement of 30-day mortality in patients who underwent ERCP within 24 h after hospitalization [15]. Accordingly, we adopted the policy of urgent ERCP as early as possible after the patient was stabilized [16]. In our regression analysis, we found a significant increase in postprocedural PEP in patients who underwent urgent ERCP for cholangitis, even though it was a small subset of patients (p = 0.018).
It is hard to define what makes an ERCP difficult and cannulation prolonged, but prolonged manipulation may damage the pancreatic duct and thus increase PEP. A similar strategy was carried out by our endoscopist surgeons and our findings confirm that as in previous studies, a difficult ERCP was significantly correlated with the incidence of PEP (p = 0.008) [17].
ERCP is commonly performed on an outpatient basis. The patients are discharged 2–4 h after follow-up if no laboratory or clinical abnormalities are detected. The patients are advised to refer to the emergency department if there is any discomfort such as abdominal pain, vomiting, or fatigue. Since pancreatitis, perforation, bleeding, and cholangitis are serious complications of ERCP, our clinical approach is to hospitalize these patients and to follow up for 24 h. In this aspect, this study differs from the literature, where the patients were under control of the clinician, and all the laboratory results were obtained in an inpatient clinic. Furthermore, emergent patients hospitalized for obstructive jaundice or cholangitis (not biliary pancreatitis) are assessed accordingly. The most commonly accepted definition of pancreatitis is an amylase level more than 3 times the upper normal limit with new or worsening abdominal pain [3, 9, 18, 19]. Post-procedural hyperamylasemia is seen in up to 75% of patients [20]. Less than 1.5 times the upper normal limit observed at 2–4 h after ERCP is not thought to indicate PEP [21]. In the present study, post-ERCP hyperamylasemia (any level, not only 3 times the upper limit) was detected at 6 h in 75 (30.8%) of 243 consecutive patients receiving first time ERCP for choledocholithiasis. The range of detected amylase levels was between 190 and 852 with a mean of 468 U/l. Only 12 (16%) of hyperamylasemia developing patients had PEP. Two (2.7%) patients with an amylase level less than 3 times the upper normal limit (226 and 294) 6 h after ERCP had PEP. Overall incidence of pancreatitis in the 243 patients was 4.9%. According to Kochar’s meta-analysis [22], PEP incidence of 108 randomized controlled trials with 13 296 patients was found to be 9.7% and 14.7% for high risk patients, with a mortality rate of 0.7%. In our series, all of the PEP patients had mild pancreatitis and responded well to I.V. fluids without anything given orally; mortality was zero. Still, cessation of oral feeding with I.V. fluid infusion is an effective treatment of PEP.
At 24 h after ERCP, the presence of abdominal pain determined the requirement for re-repeat laboratory values; otherwise, the patients were discharged. The patients who experienced abdominal pain also had increased levels of amylase, CRP and WBC. This is likely due to pancreatic inflammation and is consistent with the literature.
In our study, a history of pre-ERCP cholangitis requiring emergent care and procedural Wirsung duct cannulation requiring contrast media opacification were found to be statistically significant factors for the development of PEP (cholangitis: p = 0.048, Wirsung cannulation: p = 0.019). Pre-ERCP cholangitis is not mentioned as a risk factor in most studies about PEP, but Wirsung cannulation, although there are inconsistent data, was found as a risk factor for PEP. Guidewire use and pancreatic duct stent placement were recommended in several studies [9, 23, 24]. However, it must be kept in mind that the number of attempts and pancreatic stents also affect the incidence rate of PEP. As a last point, in the latest literature the data about the follow-up timing for the development of PEP is scarce. However, when we evaluate studies which suggest that amylase/lipase levels obtained at the 4th h may predict PEP, a common method of prediction suggested in the last 15–20 years, we see that the physical evaluation may not be meaningful at this post-procedural time [25, 26]. Our study reveals that abdominal evaluation of pain is meaningful at the 12th h.
Conclusions
Amylase level increase was most apparent 12 h after ERCP and significantly higher (p < 0.001) for the development of PEP. The first abdominal pain evaluation and examination is meaningful at the 12-hour timepoint because insufflation during the procedure and other causes of abdominal pain may result in misinterpretation.