Introduction
Oesophago-gastric cancer (OGC) is the fourth most common malignant disease and the second leading cancer-related mortality worldwide, accounting for 8% of all new cancer cases and 10% of the total cancer deaths worldwide [1]. Substantial geographic variation exists in incidence, with the highest incidence rates occurring in Asia, South America, and Eastern Europe [2]. Based on the Surveillance, Epidemiology, and End Results (SEER) database, more than 60% of OGC cases are diagnosed at the age of 65, and about 30% of these patients are older than 75 years. Additionally, the elderly population is increasing worldwide, and life expectancy has also consistently increased in most countries [3]. As a result, it is urgently necessary to define the best treatment strategy for elderly mOGC patients.
Currently, most of these patients are diagnosed with locally advanced or metastatic OGC. Although chemotherapy remains the backbone of treatment for metastatic oesophago-gastric cancer (mOGC) resulting in superior survival outcomes compared with best supportive care [4, 5], the prognosis of mOGC patients remains poor with median survival less than 1 year. Therefore, novel treatments for mOGC are clearly needed. During the past years, the emergence of molecularly targeted agents (MTAs) has provided a new promising treatment for mOGC patients [6–8]. Currently, trastuzumab and ramucirumab have been approved by the FDA for use in mOGC patients. Additionally, several novel MTAs have been extensively assessed in clinical trials. However, due to the stringent enrolment criteria for patients in prospective trials, the enrolled elderly patients in clinical studies are not entirely representative of the overall elderly patient population. Thus, clinicians should be cautious when applying these data to the overall elderly patient population. As the elderly population increases, it is urgently necessary to define the best treatment strategy for elderly mOGC patients. In the present study, we performed a meta-analysis of prospective clinical trials to investigate the efficacy of MTAs in the treatment of mOGC in this setting.
Material and methods
Selection of studies
We searched the PubMed (data from January 2000 to January 2017), Embase (data from January 2000 to January 2017) and the Cochrane Library electronic databases for relevant articles by using the following key words: “mOGC”, “gastric cancer”, “gastric carcinoma”, “esophagogastric carcinoma”, “molecular targeted agents”, “angiogenesis inhibitors”, “anti-HER agents”, “EGFR monoclonal anti-bodies”, “randomized controlled trials” and “prospective trials”. The reference lists of the retrieved articles were hand searched to identify additional relevant articles. Two authors (H.L. and L.Z.) carried out the search independently. No language restrictions were set in the search. If more than one publication was found for the same trial, the most complete, recent, and updated report of the clinical trial was included in the meta-analysis. Clinical trials that met the following criteria were included: (1) prospective randomized controlled phase II or III trials in mOGC patients (including gastric cancer or oesophagogastric carcinoma; and (2) available survival data of MTAs in elderly mOGC patients.
Data extraction
Two authors (Q.H. Z. and D.S.Z.) independently extracted the data from included trials. We conducted this meta-analysis based on the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) statement [9]. Disagreements between investigators were resolved by discussion and consensus. A standardized Excel file was used for data extraction. The following data were extracted: first author, publication year, the number of enrolled patients and elderly patients, median age, hazard ratios (HRs) with 95% confidence intervals (CIs) for OS and PFS in elderly patients.
Clinical end point and statistical methods
The outcome measures of interest were progression-free survival (PFS) and overall survival (OS). We investigated the overall efficacy of MTAs in the treatment of elderly patients with mOGC based on the data from the included trials. PFS and OS were considered as time-to-event variables, and therefore were expressed as HRs with 95% CIs for each study. HR > 1 reflected more deaths or progression in the MTA-containing regimen group, and vice versa. Heterogeneity across the studies was assessed using the χ2-based Q statistic [10]. The I 2 statistic was also calculated to quantitatively evaluate the degree of inconsistency between trials. In addition, we performed subgroup analysis based on treatment line and specific drugs to investigate the sources of heterogeneity. We used the Begg and Egger tests to assess the presence of publication bias [11]. Study quality was assessed using the Jadad five-item scale that included the randomization, double blinding, and withdrawals; the final score was reported between 0 and 5 [12]. All p-values of less than 0.05 were considered statistically significantly. All statistical analysis was calculated using Version 2 of the Comprehensive MetaAnalysis program (Biostat, Englewood, NJ).
Results
Search results
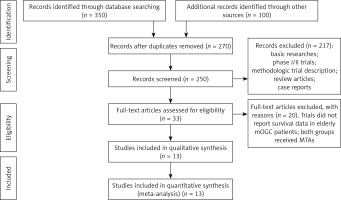
Our search yielded 250 clinical studies relevant to MTAs in mOGC patients. After reviewing the title or abstract, a total of 13 prospective randomized controlled trials were included for analysis, included 3 phase II [13–15] and 10 phase III RCTs [16–25]. The flowchart showing the study selection process is shown in Figure 1.
Characteristics of the included studies
In total, a total of 2,149 elderly patients were included in the present study. The characteristics of patients and studies are listed in Table I. Among these studies, five trials assessed angiogenesis inhibitors in mOGC patients [15, 16, 19, 22, 24], four trials assessed anti-EGFR agents in mOGC patients [13, 14, 17, 18], three trials assessed anti-HER2 agents in mOGC patients [20, 21, 25] and the remaining one trial investigated everolimus in mOGC patients [23]. Additionally, six trials assessed the role of MTAs as first-line treatment for mOGC patients, and the remaining seven trials in the second-line setting. The clinical characteristics were generally balanced between the intervention and control arm. The quality of each included study was roughly assessed according to the Jadad scale; the median Jadad score of the included studies was 5 (range: 3–5).
Table I
Baseline characteristics of 13 included randomized controlled trials
Progression-free survival
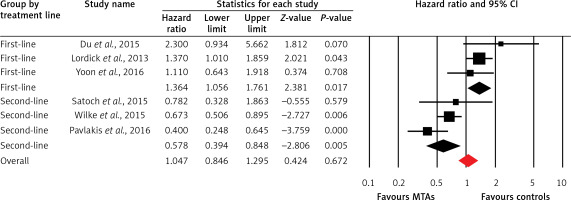
Six trials of the thirteen trials reported PFS data in the study patients. The pooled results of these studies indicated that the MTA-containing regimens did not improve PFS, giving HR = 1.05 (95% CI: 0.85–1.30, p = 0.67, Figure 2), compared with non-MTA-containing regimens. Begg’s test and Egger’s test revealed no evidence of obvious publication bias (p = 0.57 and p = 0.83, respectively). Meanwhile, significant heterogeneity was identified (I 2 = 81.5 %, p < 0.001), and the pooled HR for PFS was determined using a random-effects model. Subsequently, we performed subgroup analyses to explore potential sources of heterogeneity. Our results demonstrated that the addition of MTAs to therapies significantly improves PFS as second-line therapy (HR = 0.58; 95% CI: 0.39–0.85, p = 0.005) in elderly patients with mOGC, but not for first-line therapy (HR = 1.36; 95% CI: 1.06–1.76, p = 0.017).
Overall survival
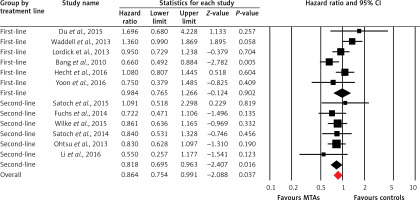
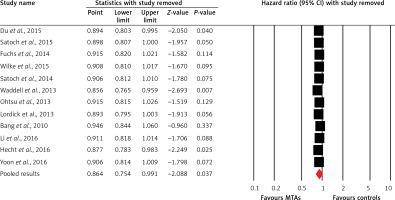
Twelve trials of the thirteen trials reported OS data of elderly patients. The pooled results demonstrated that MTA-containing regimens significantly improve OS in comparison with non-MTA-containing regimens (HR = 0.86, 95% CI: 0.75–0.99, p = 0.037, Figure 3) using a random-effects model. Begg’s test and Egger’s test revealed no evidence of obvious publication bias (p = 0.78 and p = 0.94, respectively). We also conducted sensitivity analysis to examine the stability and reliability of pooled HRs by sequential omission of individual studies. The results indicated that the significance estimate of pooled HRs was significantly influenced by omitting each single study conducted by Bang et al. [20], Fuchs et al. [19] and Ohtsu et al. [23] (Figure 4). We then performed sub-group analysis based on treatment line, and found that trials using MTA-containing regimens as second-line (HR = 0.82, 95% CI: 0.70–0.96, p = 0.016) significantly improved OS compared to non-MTA-containing regimens, but not for first-line therapies (HR = 0.98, 95% CI: 0.76–1.27, p = 0.90). Moreover, subgroup analyses identified statistically significant improvement in OS in the subgroup of elderly mOGC patients treated with angiogenesis inhibitors (HR = 0.78, 95% CI: 0.62–0.97, p = 0.027), while the use of anti-EGFR monoclonal antibodies (HR = 1.14, 95% CI: 0.90–1.44, p = 0.27), anti-HER2 agents (HR = 0.84, 95% CI: 0.61–1.17, p = 0.30) and mTOR inhibitors (HR = 0.83, 95% CI: 0.63–1.10, p = 0.19) did not significantly improve OS compared to controls.
Discussion
In the past years, the introduction of novel agents targeting specific growth and survival pathways represents the most promising treatment strategy to improve outcome for patients with mOGC [26]. A previous meta-analysis conducted by Ciliberto et al. [27] showed that the use of targeted agents significantly improved survival (OS: HR = 0.823; PFS: HR = 0.762) compared to conventional treatments in advanced gastric cancer patients, and it did not increase severe toxicities related to MTAs. Recently, Jemal et al. [28] also found that there was a significant survival benefit with targeted agents in gastric cancer patients (HR = 0.88, 95% CI: 0.79–0.99, p = 0.032) compared to controls. However, there are limited data specifically focusing on the efficacy of targeted agents in elderly patients with mOGC. As a result, we performed the present study to investigate the overall efficacy of MTAs in the treatment of elderly mOGC patients.
Our systematic review is, as far as we know, the first systematic review to specially assess the efficacy of MTAs in the treatment of elderly mOGC patients. Our study included a total of 2,149 elderly patients with mOGC from thirteen RCTs. Our results demonstrate that MTA-containing treatment shows promise as being effective for elderly mOGC patients in terms of OS. However, there is significant heterogeneity among included studies when analyzing the above endpoints. One possible explanation for this heterogeneity is that our study pooled studies across different lines of therapy investigating MTAs with different modes of action (ramucirumab, lapatinib, regorafenib, nimotuzumab, panitumumab, and cetuximab). This explanation is confirmed by the observation that heterogeneity among trials becomes non-significant when pooling the results according to treatment line and targeted agents. Pre-defined subgroup analyses indicate that the most consistent survival benefit is found when MTAs are used in second-line treatment for these patients. Additionally, greater OS benefit is observed in elderly mOGC patients treated with angiogenesis inhibitors compared to anti-EGFR monoclonal antibodies, anti-HER2 agents and mTOR inhibitors. One possible explanation for this finding is that several angiogenesis inhibitors have been proven to improve outcome of previously treated mOGC patients. Indeed, two angiogenesis inhibitors, regorafenib and ramucirumab, have been approved by the FDA as second-line treatment for the treatment of mOGC patients. However, no other MTAs, except for anti-HER2 agents, have been approved for the treatment of mOGC patients. Therefore, our findings further confirm that the effect of angiogenesis inhibitors on OS is not different in younger and older patients undergoing second-line treatment. Angiogenesis inhibitors could be recommended as second-line treatment for elderly mOGC patients.
Several limitations exist in this analysis. First of all, this is a meta-analysis at the study level. We could not obtain individual patient data from the publications; thus we could not incorporate patients’ variables into the analysis. Second, there is considerable heterogeneity among the included studies, because different targeted agents are included for analysis, although we pooled subgroup analysis according to treatment line and regimens. Furthermore, it remains undetermined which targeted agent would be the best choice for elderly mOGC patients. Finally, as positive clinical trials are more likely to be published than negative clinical trials, clinicians should pay more attention to publication bias in meta-analyses of published studies. In the present meta-analysis, we detected no publication bias using Begg and Egger tests for OS and PFS.
In conclusion, the findings of this study suggest that the MTA-containing therapies significantly improve OS but not for PFS in elderly mOGC patients. Improved efficacy is only observed in the second-line setting and not in the first-line setting. Greater OS benefit is observed in elderly mOGC patients treated with angiogenesis inhibitors compared to anti-EGFR monoclonal antibodies, anti-HER2 agents and mTOR inhibitors. Our findings support the use of angiogenesis as second-line treatment for elderly mOGC patients.