Introduction
Primary immunodeficiencies (PIDs) are a heterogeneous group of rare diseases currently comprising > 400 distinct disorders with 430 different gene defects listed [1]. PIDs affect all parts of the immune system, are mainly characterized by increased susceptibility to infections and can be associated with autoimmune diseases [2]. The largest subgroup of PIDs is characterized by impaired B-cell development and/or maturation resulting in antibody deficiency. This PID subgroup is treated with intravenous or subcutaneous immunoglobulin G (IgG) replacement therapy to prevent severe infections and organ damage [2]. Early-diagnosed, IgG-treated PID patients can expect an almost normal life span; however, morbidity is substantially increased as compared to healthy controls [3]. There is a broad consensus that quality of life (QoL) is significantly decreased in PID patients even when compared to patients with other chronic diseases [4].
Various instruments are commonly used in clinical settings to assess QoL. On one hand, generic, non-disease-specific QoL assessment tools measure health status applicable to all populations. Short Form-36 (SF-36) is a frequently used generic questionnaire which contains 36 items. It results in eight health dimensions providing two summary measures, i.e. the physical component summary (PCS) and the mental component summary (MCS) [5, 6]. On the other hand, disease-specific tools are used to assess specific aspects of chronic diseases and their treatment options. For PID, the Life Quality Index (LQI) is a well-established questionnaire evaluating QoL and treatment satisfaction in IgG replacement therapy. It contains 15 items and results in four domains providing one summary measure, i.e. the LQI index [7, 8].
Stress, recurrent infectious episodes, social determinants, and chronic health issues negatively affect QoL in PID patients [9]. All of these factors can potentially be improved by patient empowerment programmes (PEP) through which people gain greater control over decisions and actions affecting their health [10]. So far, no PEP has been established for PID patients. Therefore, a working group to develop and evaluate a primary immunodeficiency-patient empowerment programme (PID-PEP) was established in 2007 in Germany by members of the Working Group Pediatric Immunology (Arbeitsgemeinschaft Pädiatrische Immunologie – API). Changes in QoL and treatment satisfaction before and 6 months after participation in PID-PEP were evaluated in 26 adult PID patients. We hypothesized that PID-PEP would significantly improve both general (SF-36) and health-related (LQI) QoL.
Material and methods
Patients
Quality of life and treatment satisfaction were assessed before and 6 months after participating in PID-PEP in 26 adult PID patients receiving life-long IgG replacement therapy. The study period was from 2009 to 2012. Baseline characteristics of the study population are summarized in Table 1.
Table 1
Baseline characteristics of the study population. Values for median (range) or total number (percentage) are shown
PID-PEP
PID-PEP is provided by a multidisciplinary team consisting of physicians specialized in PID, psychologists, and nurses for patients with PID and IgG replacement therapy. The programme is offered near German immunodeficiency centres during a weekend group training course. Between 4 and 7 patients, as well as their relatives, participate in each course. PID-PEP consists of three main modules, i.e.I) Team building and goal definition, V) Empowerment and coping strategies, and VI) Everyday transfer of strategies learned. Furthermore, the three disease-specific modules are: II) Education on chronic disease (PID), III) Competences and motivation for long-term therapy, and IV) Management of acute and emergency situations. A more detailed description of PID-PEP is given in the Appendix. The German curriculum of PID-PEP is available online [11]. The study was approved by the ethics committee of Hannover Medical School and all subjects gave written informed consent before participating.
Evaluation of SF-36, visual analogue scale, and LQI before and at 6 months after PID-PEP
SF-36 is a well-known, easy-to-use, validated, generic multi-item scale instrument for adults measuring eight health dimensions, i.e. 1) physical functioning (PF), 2) role limitations because of physical health problems (RP), 3) bodily pain (BP), 4) social functioning (SF), 5) general mental health (MH, psychological distress and psychological well-being), 6) role limitations because of emotional problems (RE), 7) vitality (VT, energy/fatigue), and 8) general health perceptions (GH) [6]. The physical component summary (PCS) and mental component summary (MCS) were calculated based on the German population norm 1994 [12]. The SF-36 health dimensions and component summaries were transformed to a 0 (worst status) to 100 (best status) scale as described [13]. Furthermore, patients were asked to indicate their present health status on a visual analogue scale (VAS) ranging from 0 (worst status) to 100 (best status).
LQI has originally been developed for patients with PID receiving home-based intravenous immunoglobulin therapy [8]. It consists of 15 items assessing the perception of the impact of IgG treatment on daily activities on a 7-point Likert scale ranging from extremely good (7) to extremely bad (1). The wording of the original LQI was changed slightly, i.e., the wording “IVIG treatment” was changed to “immunoglobulin treatment”. The 15 items were summarized in four domains, i.e. I) treatment interference, II) therapy-related problems, III) therapy setting, and IV) treatment costs. Furthermore, the LQI index was calculated as a summary measure over all 15 items. The LQI domains and summary measure were transformed to a 0 (worst status) to 100 (best status) scale as described [7].
Results
VAS and MCS values are significantly improved after PID-PEP
Median (range) VAS values of present health status significantly increased from 68 (10-98) at baseline to 76 (35-98) 6 months after PID-PEP (Table 2, p = 0.002). The SF-36 summary measure MCS significantly improved from 36 (20-53) to 43 (13-55) following the programme (Table 2, p = 0.042). The SF-36 summary measure PCS numerically increased by 3 points; however, this improvement did not reach statistical significance (Table 2, p = 0.150). Of the eight SF-36 dimensions, VT significantly improved from 48 (20-65) at baseline to 55 (15-70) at 6 months after PID-PEP (Table 2, p = 0.025). Furthermore, there were nonsignificant (p < 0.10) improvements within role-physical (RP, baseline: 38 [0-100], at 6 months: 75 [0-100], p = 0.052) and MH (baseline: 64 [36-76], at 6 months: 68 [28-80], p = 0.081) (Table 2). No significant changes were observed within the SF-36 dimensions PF, BP, SF, role-emotional (RE), and GH (Table 2). PID patients at baseline showed numerically lower scores in all eight dimensions of the SF-36 as compared to a normal German population-representative control group (Fig. 1).
Fig. 1
Mean SF-36 dimensions in a German population-representative study [14], as well as in all patients (n = 26) before and 6 months after PID-PEP
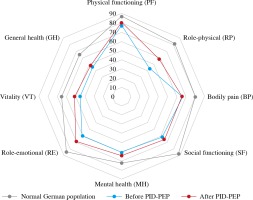
Table 2
VAS and SF-36 scale values in all patients (n = 26) before and 6 months after PID-PEP. Values for median (range) are shown. Statistical significance was tested by Wilcoxon signed-rank test and p-values are given
LQI index, as well as LQI domains I and II, are significantly improved after PID-PEP
Median (range) LQI index significantly increased from 77 (36-98) at baseline to 86 (44-99) 6 months after PID-PEP (Table 3, p = 0.008). Furthermore, the LQI domains treatment interference (I) and therapy-related problems (II) significantly improved from 78 (39-100) to 88 (39-100) (p = 0.008) and 73 (25-100) to 83 (38-100) (p = 0.003), respectively (Table 3). The LQI domains therapy setting (III) and treatment costs (IV) were unchanged after PID-PEP (Table 3).
Table 3
LQI scale values in all patients (n = 26) before and 6 months after PID-PEP. Values for median (range) are shown. Statistical significance was tested by Wilcoxon signed-rank test and p-values are given
Discussion
To the best of our knowledge, this is the first study to assess changes in QoL following participation of PID patients in a patient empowerment programme (PID-PEP). We report for the first time that the VAS score of present health status, MCS of the SF-36, and LQI index were significantly improved following PID-PEP.
All eight dimensions of the SF-36 were numerically lower in PID patients at baseline as compared to a normal German population-representative control group [14], indicating that QoL is significantly decreased in PID patients due to the severity of their chronic disease. In accordance with our findings, all eight SF-36 dimensions were also lower in Italian patients with common variable immunodeficiency disease (CVID) as compared to healthy subjects [4]. In the same CVID population, SF-36 scores for RP and GH were lower as compared to other chronic diseases including diabetes mellitus, cancer, chronic obstructive pulmonary disease, and mental disorders [4]. It is interesting to note in this context that mean RP values at baseline in our patient cohort were particularly low as compared to a normal German control group.
Of the eight SF-36 dimensions, six dimensions numerically improved and two were unchanged 6 months after PID-PEP as compared to baseline with VT, as well as MCS and VAS of present health status, reaching statistical significance. These findings suggest that improvements in QoL parameters are possible by a multidimensional PEP at least over a period of 6 months. It is important to consider in this context that QoL decreases over the natural course of a chronic disease. For CVID, Tabolli and co-workers demonstrated a significant decrease in five SF-36 dimensions, i.e. PF, BP, GH, SF, and RE, during an observation period of 6 years [4].
The LQI index significantly improved six months after PID-PEP as compared to baseline. The LQI is a well-known tool to evaluate treatment satisfaction for IgG replacement therapy in four domains [7]. In our patients, the domains treatment interference with daily life activities (I) and therapy-related problems (II) were significantly improved 6 months after PID-PEP. These improvements most likely reflect the focus of PID-PEP on motivation, education, and everyday transfer, next to practical issues of living with a chronic disease demanding lifelong therapy.
Well-being of patients has played a more central role in patient-centred clinical medicine in recent years [10]. This development does not substitute but adds to more traditional endpoints including mortality and morbidity. The present study provides evidence that PID-PEP improves the impact of chronic disease on health-related QoL in several domains over a period of 6 months. Since PID-PEP is not regularly covered by public and private health insurance companies, the present beneficial results should support informed decision making for health care providers. Cost-benefit analyses need to be performed to elucidate the cost-effectiveness of PID-PEP.
A strength of our study is the relatively long follow-up period of 6 months, implying that PID-PEP has sustainable effects on general and health-related QoL. Limitations include the lack of a control group, e.g. PID patients on a waiting list or only receiving written advice. Therefore, beneficial effects of PID-PEP on QoL cannot be proven by the present study; however, QoL parameters tend to decrease in CVID patients over time [4]. Due to the rarity of PID, the number of patients is limited. Since several general and health-related QoL parameters are significantly improved 6 months after PID-PEP, further studies in larger patient samples and with adequate control groups, as well as over longer-term follow-up, are warranted.
Taken together, it is shown for the first time that a patient empowerment programme for PID (PID-PEP) significantly improves general and health-related QoL. It needs to be evaluated in future studies whether the beneficial effects of PID-PEP are sustained over longer periods of time and whether repeated PID-PEP sessions further improve QoL outcome.


