Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
OSTEOPOROSIS / BASIC RESEARCH
A study on the mechanism of Wnt inhibitory factor 1 in osteoarthritis
1
Department of Sports Medicine and Joint Surgery, The People’s Hospital of China Medical University, Shenyang, Liaoning, China
Submission date: 2017-07-15
Final revision date: 2017-11-01
Acceptance date: 2017-11-26
Online publication date: 2020-06-01
Publication date: 2020-05-26
Arch Med Sci 2020;16(4):898-906
KEYWORDS
TOPICS
ABSTRACT
Introduction:
In our study we aimed to investigate the mechanism of Wnt inhibitory factor 1 (WIF1) on regulating chondrocyte proliferation and apoptosis via reactive oxygen species (ROS) and the Wnt/β-catenin signaling pathway in osteoarthritis (OA).
Material and methods:
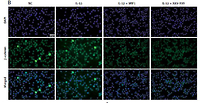
Osteoarthritis chondrocytes were treated with interleukin 1β (IL-1β) to simulate an inflammatory condition. Quantitative real-time polymerase chain reaction (qRT-PCR) and western blot were applied for detecting WIF1 expression in OA chondrocytes. MTT assay and flow cytometry were carried out to analyze the cell proliferation and apoptosis. Content of ROS was detected using flow cytometry, and activity of the Wnt/β-catenin signaling pathway was detected using immunofluorescence, western blot and luciferase reporter assay. Western blot and enzyme-linked immunosorbent assay (ELISA) were performed to detect the expression of apoptosis-related proteins and secretion of matrix metalloproteinases (MMPs).
Results:
WIF1 expression in OA chondrocytes was significantly lower than in normal chondrocytes. After WIF1 cDNA transfection, the aberrantly high ROS level in OA chondrocytes was down-regulated, which led to the increase of proliferation and reduction of apoptosis. The Wnt/β-catenin signaling pathway was suppressed by WIF1 overexpression and the secretion of MMPs was therefore reduced.
Conclusions:
Up-regulation of WIF1 would promote proliferation and suppress apoptosis of OA chondrocytes through eliminating ROS production and reduce secretion of MMPs via blocking the Wnt/β-catenin signaling pathway.
In our study we aimed to investigate the mechanism of Wnt inhibitory factor 1 (WIF1) on regulating chondrocyte proliferation and apoptosis via reactive oxygen species (ROS) and the Wnt/β-catenin signaling pathway in osteoarthritis (OA).
Material and methods:
Osteoarthritis chondrocytes were treated with interleukin 1β (IL-1β) to simulate an inflammatory condition. Quantitative real-time polymerase chain reaction (qRT-PCR) and western blot were applied for detecting WIF1 expression in OA chondrocytes. MTT assay and flow cytometry were carried out to analyze the cell proliferation and apoptosis. Content of ROS was detected using flow cytometry, and activity of the Wnt/β-catenin signaling pathway was detected using immunofluorescence, western blot and luciferase reporter assay. Western blot and enzyme-linked immunosorbent assay (ELISA) were performed to detect the expression of apoptosis-related proteins and secretion of matrix metalloproteinases (MMPs).
Results:
WIF1 expression in OA chondrocytes was significantly lower than in normal chondrocytes. After WIF1 cDNA transfection, the aberrantly high ROS level in OA chondrocytes was down-regulated, which led to the increase of proliferation and reduction of apoptosis. The Wnt/β-catenin signaling pathway was suppressed by WIF1 overexpression and the secretion of MMPs was therefore reduced.
Conclusions:
Up-regulation of WIF1 would promote proliferation and suppress apoptosis of OA chondrocytes through eliminating ROS production and reduce secretion of MMPs via blocking the Wnt/β-catenin signaling pathway.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.