Introduction
One of the global diseases threatening human life is hydatid disease, which is caused by tapeworms of the species Echinococcus granulosus [1]. The liver is the most common organ which is infected by this disease (50–70%) [2]; the second most common organ after the liver is the lung (20–30%), and the other organs less commonly affected are the spleen, kidneys, heart, bones, central nervous system and other organs [3]. Being asymptomatic is one of the dangerous characteristics of this disease, so the size of the cysts can be increased and after many years they can rupture, which results in anaphylactic shock [4, 5]. This disease is much more common in endemic regions of countries including Asia, the Middle East, Australia, Mediterranean countries, Europe, and South America; and the rate of incidence is annually more than 50 out of 100,000 persons according to the report of the World Health Organization (WHO) [1, 6, 7]. To diagnose this disease we need to combine some techniques such as imaging, histopathology, nucleic acid detection, and serology [8]. After diagnosing this disease, the physician needs to consider an approach from three known treatments including open surgery, PAIR (Puncture, Aspiration, Injection and Re-aspiration) and laparoscopic surgery (Lap) for hydatid liver cysts [3].
Open surgery, which is used for a complete removal of cysts, is a formal and traditional procedure among the surgeons for treating this disease [1, 9]. The PAIR procedure is considered one of the noninvasive treatments [10]. This procedure was first presented by Mueller in 1985 [11]. Although this procedure is safe, effective, cheap and easy to perform, it is controversial from the perspective of the association of surgeons [12]. The controversy refers to the disadvantages of this procedure, since it can cause anaphylactic shock, death, and intraperitoneal spillage while performing puncture [13]. However, it has recently been reported that PAIR is the accepted treatment of uncomplicated liver hydatid cysts in the stages of CE1 and CE3a [8].
Laparoscopic surgery is a new surgical technique for liver hydatid cysts that require incision (0.5–1.5 cm) [14] and was first performed in 1992 [15]. Due to the benefits of this procedure such as small incisions and less postoperative pain, most surgeons might choose it as their first choice, but it can be challenging because of some disadvantages such as being expensive, requiring special devices and experience, being suitable for selected cases, intraperitoneal spillage and shock [15].
There have been many advances and scientific reports on several topics related to systematic review and meta-analysis which are of much interest to evaluate and derive useful information from the outcomes of various approaches in treating diseases in research areas in terms of their clinical efficacy, diagnostic accuracy, and controlling incidence of particular diseases in local or global areas over the world [16–20]. According to the abovementioned information, although WHO confirmed PAIR treatment in some stages, there is a challenge in the selection of new hydatid liver cyst treatment procedures (i.e., laparoscopic surgery and PAIR). To the best of the authors’ knowledge, this study is the first time that the two approaches have been compared by performing a systematic review and meta-analysis. Furthermore, based on the PICO (Patients, Intervention, Comparison, Outcomes) statement, the current systematic review and meta-analysis achieves its objective by defining patients who are infected by liver hydatid cysts, the intervention includes the PAIR and Lap procedures to treat the disease, the comparison element answers the question for comparing these procedures in terms of their outcomes, and finally the outcomes of interest consist of cure, postoperative complications, mortality, and recurrence rates of the intervention procedures which need to be increased, decreased, decreased and decreased, respectively.
Material and methods
Based on the PRISMA (Preferred Reporting Items for Systematic Reviews) guidelines [21], the databases were searched, and the articles related to PAIR and laparoscopic surgery of liver hydatid cyst (conservative and radical) were derived and reviewed.
Search method
We systematically searched and reviewed two databases, PubMed and Scopus, during the period of January 2000 to December 2016, in order to select and evaluate the relevant published articles.
Data search and extraction
The Boolean query used for searching the abovementioned databases was as below: ([liver OR hepatic] AND [Echinococcosis OR hydatid]) AND (laparoscop* OR PAIR OR [puncture OR percutaneous] AND aspiration AND injection AND re-aspiration) OR ultrasound.
Inclusion and exclusion criteria
Inclusion criteria included the English language, retrospective and prospective studies, and some comparative studies of PAIR and laparoscopic treatment research.
Exclusion criteria were as follows: (i) unrelated articles, (ii) studies with inadequate data, (iii) non-English language, letters and case report articles, (iv) systematic review, meta-analysis, and duplicated articles. Reported data on the incidence of recurrence, mortality, cure rate and post-procedural complications were stored in an Excel file according to the checklist.
Two researchers independently and separately extracted the data from each study. Extracted data were sample size, mean ages, gender, type of procedures, minor and major complications, recurrence, cure rate, mortality, length of hospital stay and time of operations and Re-PAIR or Re-operation. Additionally, the terms heterogenicity (I²) and publication bias were analyzed in both groups.
Patients’ characteristics
Liver hydatid cysts can be confirmed by ultra-sonography, abdominal computed tomography (CT) scans, and serologic methods. Patients whose ages were in the range from 5 to 87 years old with uncomplicated liver hydatid cysts defined as intact non-infected liver hydatid cysts with no biliary system or other viscera communication were considered.
All patients with complicated liver hydatid cysts with clinical signs and suspicion of biliary system or other viscera communication were not selected for any type of treatment (PAIR or Lap).
Interventions
PAIR procedure
The PAIR procedure is known as a noninvasive treatment (10). To perform this procedure, cysts are first diagnosed by ultrasound guidance. Then, the cyst is punctured percutaneously by local anesthesia. After that, cystic fluid is aspirated, and scolicidal agents (hypertonic saline, alcohol, Betadine or cetrimide, and others) are injected into the cyst cavity. Finally, after 20 to 30 min the injected solution is re-aspirated [22].
Laparoscopic procedure
This method is done by laparoscopic instruments, general anesthesia and evacuating hydatid fluid using scolicidal agents with soaked scolicidal gauzes. Then, pericystectomy, omentoplasty, and biliary opening closure in patients with uncomplicated liver hydatid cysts are performed.
Outcome measures
Successful clinical outcomes are assessed by disappearance of hydatid cysts as well as their cure rates. Postoperative complications, mortality, and recurrence rates of the two abovementioned procedures are taken as clinical hazards. Hospital stays and operative times of the patients, quality of life, and health economics were not recognized as serious events to be meta-analyzed.
Postoperative complications are categorized as minor (i.e., skin rash, pruritus, and anaphylaxis) and major (i.e., infections abscess, bleeding, seeding of cyst communication with intra-biliary system or the need to perform the re-operation or open surgery).
Analysis
Eligible studies were extracted into a spreadsheet file for analysis. Pooled analysis was performed on studies to calculate event rates. Event rates were used as the effect measure estimate. Meta-analysis was performed with a random effect model. The number of included published articles was 57 (23 for PAIR and 34 for laparoscopic surgery studies). The measures of analysis were postoperative complications, mortality, recurrence, and cure rates of both types of procedures.
We assessed publication bias using funnel plots and Egger’s tests. The heterogeneity test was done by quantitative measures including Q, p-value, and I². In the presence of heterogeneity, meta-regression was performed and using (R²) we could determine if published year or sample size explain the heterogeneity or not.
After generating the funnel plots and performing the required regression modeling such as interception of Egger’s regression tests and their p values, the publication bias of the study was assessed. Based on various studies for assessing publication bias a p < 0.05 is regarded as significant. The statistical analysis done on all data was performed using both meta-analysis developed in Mashhad University of Medical Sciences (meta-MUMS) and Comprehensive Meta-analysis (CMA) version 2.2.0.064 [23] while only our implemented software results are presented in this study to show our tool to be an alternative means for CMA in future studies of researchers.
A p < 0.05 for the heterogeneity test or I² > 50% indicated significant heterogeneity among the studies. In this study, all of the results and their statistical analyses are calculated and obtained from the meta-MUMS tool.
Results
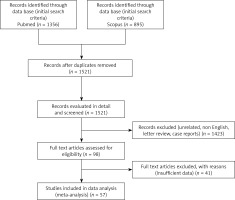
The potentially relevant studies from initial search criteria returned 2251 titles. Figure 1 represents the literature review search based on PRISMA guidelines. a total of 2832 patients within 57 studies met our inclusion criteria, 34 studies for laparoscopy [3, 5, 9, 15, 24–53] with n = 1182, and 23 studies for the PAIR approach [3, 54–75] with n = 1650 and therefore were analyzed to evaluate the effects of laparoscopy and PAIR techniques on the outcome of treatment of liver hydatid cysts. Of 57 studies, 17 took place in Turkey, 11 in India, 5 in China, 2 in Croatia, 2 in Tunisia, 2 in Chile, 2 in Spain, 2 in Russia, 2 in Ukraine, 2 in Italy, 1 in Romania, 1 in Saudi Arabia, 1 in Pakistan, 1 in Lebanon, 1 in Argentina, 1 in Iraq, 1 in The Netherlands, 1 in Yugoslavia, 1 in Denmark, and 1 in Bosnia Herzegovina. One study [3] reported on both PAIR and laparoscopic groups. It is worth mentioning that the relation of the cysts to the biliary tree is important in the PAIR procedure due to preventing the sclerosing cholangitis effect of some injected scolicidal agents which will be diagnosed by aspiration. However, all studies included in this research followed this principle.
Six (of 57) studies [26, 27, 37, 39, 40, 52] were prospective and the remaining ones were retrospective studies. Ages and operating times of the two procedures and hospital stays are not mentioned clearly in some studies, so they were not analyzed. The information from the extracted articles from the databases based on the Boolean query where the Lap and PAIR treatments of liver hydatid cysts are included is listed in Tables I and II. Moreover, Tables I and II show the characteristics, demographics, type of procedures, and outcomes of the two abovementioned procedures. Also, Figure 2 displays forest plots of these two procedures.
Table I
Literature review studies involving 1650 patients (2127 cysts) with liver hydatid cysts and clinical outcomes undergoing PAIR procedure
Table II
Literature review studies involving 1182 patients with liver hydatid cyst and clinical outcomes undergoing laparoscopic surgery
Figure 2
Forest plots of two procedures (A, C, E, G – forest plots of cure rates, postoperative complications, mortalities, and recurrences of PAIR group; B, D, F, H – forest plots of cure rates, postoperative complications, mortalities,and recurrences of Lap procedure)




The random effect meta-analysis of cure rate outcomes in PAIR and laparoscopy procedures are as follows:
Random effect meta-analysis of PAIR: event rate = 0.928, 95% CI lower limit = 0.89, upper limit = 0.953, Z = 10.951, p < 1e–16. The forest plot is illustrated in Figure 2 A.
Random effect meta-analysis of laparoscopy: event rate = 0.907, 95% CI lower limit = 0.859, upper limit = 0.940, Z = 9.431, p < 1e–16. The forest plot is illustrated in Figure 2 b.
The inconsistency and heterogeneity parameters can also be estimated by quantitative measures in both groups. The results of the heterogeneity test of the PAIR procedure are Q = 75.067, df = 22, p = 1.031e–7, I² = 70.693, Γ² = 0.706, and hence it has high heterogeneity. The results of the heterogeneity test of the laparoscopy procedure are Q = 122.575, df = 33, p = 3.133e–12, I² = 73.078, Γ² = 1.203, and therefore it also has high heterogeneity. However, in both heterogeneity tests, the p-values were significant.
Meta-regression was performed using a random effect model in the PAIR procedure based on the published years and sample sizes of the studies. In the meta-regression of the “published year”, slope = 0.00028, SE = 0.04716, p = 0.99535, Γ² = 0.75470, p-value is not significant so there is no relation between published year and PAIR cure rate (Figure 3 A) but in the meta-regression of the sample size slope = 0.00516, SE = 0.00215, p = 0.01625, Γ² = 0.43135 (Figure 4 A), which means that the greater the sample size, the higher the cure rate.
Figure 3
Meta-regression of two procedures: relation between (log cure, complications, and recurrences) and published year (A, C, E) for PAIR procedures (B, D), and Lap procedures

Figure 4
Meta-regression of two procedures: relation between (log cure, complications, and recurrences) and sample sizes (A, C, E) for PAIR procedures (B, D), and Lap procedures

Additionally, the meta-regression was performed by the random effect model in the Lap procedure based on published years and sample sizes of the studies. In the meta-regression of “published year”, slope = –0.0234, SE = 0.04769, p = 0.624, Γ² = 1.2862 (Figure 3 B). The p-value is not significant so there is no relation between published year and cure rate of the Lap procedure. However, in the meta-regression of sample size, slope = 0.0239, SE = 0.00945, p = 0.01136, Γ² = 0.98179 (Figure 4 B). The sign of the slope and significance of the p-value show that there is a direct relationship between sample size and cure rate.
Sample size of PAIR and Lap procedures can explain 39% and 18.4% of heterogeneities while published year cannot explain the heterogeneity in either procedure.
The funnel plots of the two procedures are shown in Figures 5 a and b. The result of the meta-analysis for publishing bias, including Egger’s regression test with intercept 0.950 and p = 0.244, does not show any significant publication biases in the PAIR approaches, while the results of Egger’s tests with intercept 2.216 and p = 0.00095 show a publication bias in laparoscopic procedure (Table III).
Table III
Egger’s tests of outcomes PAIR and laparoscopy procedures in the treatment of liver hydatid cysts
Figure 5
Funnel plots of two procedures (A, C, E, G – funnel plots of cure rates, postoperative complications, mortalities, and recurrences of PAIR group; B, D, F, H – funnel plots of cure rates, postoperative complications, mortalities, and recurrences of Lap procedure)

The meta-analysis random effect model indicates that the cure rate of PAIR is better than the laparoscopic procedure (92.8% vs. 90.7%, respectively).
Postoperative complications
Figures 2 C and D display forest plots of these two procedures. Results of random effect meta-analysis of postoperative complications in PAIR and laparoscopy procedures are as follows: Random effect meta-analysis of PAIR procedure: event rate = 0.185, 95% CI lower limit = 0.122, upper limit = 0.271, Z = –5,938, p = 2.89e–9. Random effect meta-analysis of laparoscopy: event rate = 0.187, 95% CI lower limit = 0.148, upper limit = 0.233, Z = –10.316, p < 1e–16.
The inconsistency and heterogeneity parameters can be predicted by quantitative measures in both groups. Heterogeneity of PAIR procedure is Q = 192.381, df = 22, p < 1e–16, I² = 88.564, Γ² = 1.102, and so it has high heterogeneity.
Meta-regression was performed using the random effect model based on published year of PAIR procedure where slope = –0.04371, SE = 0.04993, p = 0.38136, Γ² = 1.207 (Figure 3 C). The p-value is not significant so there is no relation between published year and PAIR complication rate. However, in thet meta-regression of sample size, slope = –0.00598, SE = 0.00307, p = 0.05130, Γ² = 1.1551 (Figure 4 C); the p-value is not significant, so there is no relationship between sample size and complication rate.
Heterogeneity of laparoscopy procedure is Q = 87.486, df = 33, p = 8.155e–7, I² = 62.208, Γ² = 0.373 and it has a medium heterogeneity.
Meta-regression based on “published year” of studies of laparoscopy procedure was performed using a random effect model (slope = 0.02821, SE = 0.02837, p = 0.32006, Γ² = 0.3948; Figure 3 D) that showed no relationship between complication rate and published year because the p-value was not significant. In sample size meta-regression studies of the laparoscopy procedure using a random effect model (slope = –0.01365, SE = 0.00539, p = 0.01141, Γ² = 0.28204; Figure 4 D) the sign of the slope and significance of the p-value show that sample size and complications are inversely related.
Published years of studies of PAIR and Lap procedures cannot explain the heterogeneities while sample size studies of PAIR and Lap procedures (R² = 0 and R² = 0.244) can explain only 24.4% of heterogeneity in Lap procedure complications.
The funnel plots of the two procedures are shown in Figures 5 C and D. The result of the meta-analysis for publication bias including Egger’s regression test with intercept 0.521 and p-value 0.698 in the PAIR approach and also the test result on laparoscopy technique with intercept 0.826 and p = 0.266 indicate no proof of publication bias in either procedure (Table III).
The meta-analysis random effect model demonstrates that the complications of PAIR procedures are lower than those of laparoscopic procedures (18.5% vs. 18.7%).
Mortality
Figures 2 E and F display forest plots of mortalities using the two procedures. The results of random effect meta-analysis of mortality in PAIR and laparoscopy procedures are as follows:
Random effect meta-analysis of PAIR procedure: event rate = 0.011, 95% CI lower limit = 0.007, upper limit = 0.02, Z = –15.935, p < 1e–16.
Random effect meta-analysis of laparoscopy: event rate = 0.018, 95% CI lower limit = 0.011, upper limit = 0.029, Z = –16.231, p < 1e–16.
The heterogeneity test result of the PAIR procedure is Q = 10.542, df = 22, p = 0.981, I² = 0%, Γ² = 0%, and the p-value of the heterogeneity test is not significant. This shows that the studies do not have the property of heterogeneity but it cannot be considered as homogeneous, and meta-regression is not needed. The heterogeneity test result of laparoscopy mortality is Q = 10.87, df = 33, p = 1, I² = 0%, Γ² = 0%. This also shows that it does not have the property of heterogeneity but it cannot be considered as homogeneous and meta-regression is not needed.
The funnel plots of the two procedures are shown in Figures 5 E and F. The result of the meta-analysis for publishing bias, including Egger’s regression test with intercept = 1.013 and p = 0.36 in the PAIR procedure, indicates no proof of publication bias, while the results of Egger’s tests with intercept = 47.433 and p < 1e–16 show publication bias in laparoscopic procedure (Table III).
The random effect meta-analysis shows that the mortality rate result of PAIR is lower than that of the laparoscopy procedure (1.1% vs. 1.8%).
Recurrence
Figures 2 G and H display forest plots of recurrences of these two procedures. Results of random effect meta-analysis of recurrence in PAIR and laparoscopy approaches are as follows:
Random effect meta-analysis of PAIR procedure: event rate = 0.05, 95% CI lower limit = 0.03, upper limit = 0.082, Z = –10.928, p < 1e–16. Random effect meta-analysis of laparoscopy: event rate = 0.039, 95% CI lower limit = 0.027, upper limit = 0.058, Z = –15.508, p < 1e–16.
The heterogeneity test result of the PAIR procedure is Q = 67.982, df = 22, p = 1.367e–6, I² = 67.639, Γ² = 0.885 and it is clear that the p-value of the heterogeneity test is significant, so the studies are heterogeneous. The heterogeneity test result of laparoscopy recurrence is Q = 41.805, df = 33, p = 0.14, I² = 21.062, Γ² = 0.281. This shows that it did not have the property of heterogeneity but it cannot be considered as homogeneous.
Results of meta-regression based on published year of the PAIR procedure using the random effect model: slope = –0.02780, SE = 0.05298, p = 0.59982, Γ² = 0.9327 (Figure 3 E). The p-value is not significant, so there is no relationship between published year and recurrence rate. Meta-regression based on sample sizes of PAIR procedure using random effect model: slope = –0.00486, SE = 0.00261, p = 0.06278, Γ² = 0.6938 (Figure 4 E). The p-value is not significant, so there is no relation between sample size and recurrence rate.
The published year and sample size meta-regression of PAIR studies cannot explain the heterogeneity.
The funnel plots of the two procedures are shown in Figures 5 G and H. The result of the meta-analysis for publication bias, including Egger’s regression test with intercept = –1.099 and p = 0.136 in the PAIR procedure and the results of Egger’s tests with intercept = –0.537 and p = 0.345 for the laparoscopic procedure indicate no proof of publication bias in either procedure (Table III).
This random effect meta-analysis shows that the recurrence rate of PAIR is higher than that of the Lap procedure (5% vs. 3.9%).
Discussion
In the current study, we aimed to determine the effects of PAIR and laparoscopic surgery procedures on the treatment of liver hydatid cysts. We conducted a meta-analysis of 6 prospective and 51 retrospective studies. Using overall event rate estimations demonstrated statistically significant effects of the PAIR procedure on cure rate, postoperative complications, and mortality. Recurrence rates for the Lap procedure are lower than for PAIR approaches. Overall, in this study we found that the PAIR procedure is a better and preferable treatment compared to laparoscopic surgery.
There is no “best” treatment option for Echinococcus granulosus, and also there are no randomized clinical trial articles in the literature that have compared these two types of treatment modalities to date. PAIR is a minimally invasive technique and has some benefits, being less risky and more cost effective compared to laparoscopic surgery [76]. PAIR intervention has been performed under ultrasonography or tomography guidance and may not identify small or undetected cysts. The aim of PAIR therapy is to destroy the germinative layer and evacuate its contents. PAIR is not suitable for all types of hydatid cysts recommended by WHO. It is recommended in inoperable patients, or those who refuse the surgery, fail to respond to ABZ alone, patients who relapsed after surgery, and in the first time treatment of stages CE1 and CE3a larger or smaller than 5 cm with ABZ therapy. It is contraindicated in lung cysts and biliary fistula and stages CE4, CE5, CE2 and CE3b [8, 59].
Laparoscopic surgery for the treatment of cystic echinococcosis is a technical option in selected and uncomplicated patients, but the risk of complications, especially spillage, has never been fully evaluated [9]. Laparoscopic surgery is also a minimally invasive procedure and successful in hepatic hydatid cysts located peripherally and anteriorly. Posterior, deep cysts, and cysts located close to the inferior vena cava and calcified hydatid cysts cannot be selected for laparoscopic surgery [9, 28, 54]. Laparoscopic intervention was performed in a visual field of view under general anesthesia. Small incisions reducing post-operative pain and shortness of hospital stay are the advantages of the Lap procedure [77]. Operative laparoscopic mortality of this study is 0% while in the literature rates up to 0.22% are reported [15], and can be raised if surgical and medical facilities are inadequate [78]. The aim of this procedure is to remove cyst contents completely. The removal of the cyst is usually concurrent with partial pericystectomy. The closed type is defined as removal of the cyst content without opening the cyst and the open type as sterilizing the cyst content and scoleces with scolicidal agents and evacuating its contents [79]. Furthermore, total cystectomy may be performed by laparoscopy in advanced laparoscopic centers [53].
In this meta-analysis although the cure rate of PAIR compared with laparoscopy is higher (ER = 0.928 vs. ER = 0.907), the complication rate is low (ER = 0.185 vs. ER = 0.187), the mortality rate is also low (ER = 0.011 vs. ER = 0.018), and it has a high recurrence rate (ER = 0.050 vs. ER = 0.039). In the study of Brunetti, it has been shown that PAIR is safe and effective for many patients with cysts of stages CE1 and CE3a [8]. This meta-analysis study confirmed the WHO protocol for treatment of liver hydatid cysts at stages CE1 and CE3a but it remains debatable whether PAIR should be recommended for WHO stages CE2 and CE3b [8, 59]. Most of the reviewed studies of this article did not report based on the stages of the WHO classification.
In the comprehensive analysis study of Chen, the Lap procedure has a higher cure rate with high complication rates [1]. Inadequate response rates were not reported in our study due to insufficient data while Smego reported 2% in the literature [76].
Indeed, PAIR was also considered a more time saving therapy than the Lap procedure.
Mean duration of hospital stay of this study in the PAIR procedure in comparison with laparoscopy is 4.2 (1–14) days vs. 5.2 (2–30) days while in the literature it is 2.2 days vs. 4.9 days. Most studies reported that the PAIR group could be discharged from hospital on the day of receiving PAIR and continue the ABZ therapy at home [3, 68].
In this study, the mean follow-up period of PAIR in comparison with laparoscopic procedures is 35.62 (6–720) months and 29.12 (4–132) months, respectively, which shows the high accuracy of recording of the recurrences.
These two procedures have different post-interventional complications. Anaphylactic reactions in this study occur much more frequently in patients with the PAIR intervention (3.5%) compared with the Lap procedure (0.17%), as confirmed by the literature [1]. After it, biliary leaks/fistula in patients with the lap procedure (1.2–4.82%) compared with patients of the PAIR group (4.4–18%) and the incidence of any type of infection in the Lap procedure in comparison to PAIR patients is high (4.06% vs. 2.12%), as confirmed by the literature [1, 76]. Failed approaches of the two abovementioned groups of this study are 0.61% in PAIR in comparison to 1.62% in the Lap procedure. It is reported as up to 23% in the Lap approach [64] and the incidence of spillage is 1.35% in the Lap and 0.1% in the PAIR procedure. This is confirmed by Smego’s study [76]. Some studies have not reported the spillages of cystic content and hydatid fluid of PAIR [67, 80].
The incidences of recurrence of this study in laparoscopy and PAIR procedures are 3.64% to 3.89% (event rates: 3.9% vs. 5%). It was reported as 0–3.3% in Lap [15] and 1.6% to 4% in PAIR approaches [64, 70, 76].
The incidence of mortality in PAIR and laparoscopic procedures of our study are 0/1650 and 3/1182 patients or 0% and 0.25% respectively (event rates: 1.1% vs. 1.8%). However, these values are reported as 0.01% to 0.9% in the literature [68, 76].
The incidence of postoperative complications in laparoscopy and PAIR procedures was 227/1182 and 300/1650 patients or 19.2% vs. 18.18% (event rates: 18.7% vs. 18.5%).
Mean hospitalization times for laparoscopic and PAIR procedures of this study were not determined due to inadequate data.
We assessed heterogeneity both graphically and quantitatively. Based on this assessment, we identified heterogeneity of the studies that may have influenced the results of our meta-analysis. In this meta-analysis, we examined cure rates, complications, mortality, and recurrences of both abovementioned procedures separately and found comparable results, which were not reported in the English language studies.
The results of the meta-analysis reported in this study should be viewed within the limitations of the included studies. Nearly all of the included studies concern selected and uncomplicated hydatid cysts of the liver. Therefore in this analysis we analyzed simple and uncomplicated forms of liver hydatid cysts. There were still several limitations: Since designing and performing prospective randomized controlled studies (RCT) studies on liver hydatid cyst treatment according to ethical issues are difficult, there were not any RCT studies in the literature to be included in our study.
Our systematic review only concerned PubMed and Scopus databases, so conference proceedings and unpublished articles were not included in our study. Hence, some valuable data may not have been considered. Any systematic review might have publication bias since it is unavoidable. In this case, we analyzed publication bias with funnel plots and Egger’s regression tests.
This meta-analysis has the ability to confirm that PAIR treatment is the best approach by reducing the mortality and complication rates and achieving higher cure rates in treating uncomplicated liver hydatid cyst.
Finally, performing more RCT studies with a sufficient sample size according to ethical issues to achieve this aim is suggested for future systematic reviews and meta-analysis studies. This can improve the results of these types of studies.
In conclusion, this study is a systematic review and meta-analysis conducted on published articles of the literature that shows a significant trend toward an advantage of PAIR for treatment of liver hydatid cyst as confirmed by the WHO protocol [8]. Surgeons, advanced laparoscopists and interventional radiologists should be aware of the information and results of this study showing a higher cure rate, lower complication rate, and lower mortality in the PAIR procedure compared with laparoscopic surgery.
The only advantage and superiority of the Lap procedure is having lower recurrence in comparison to the PAIR procedure.