Introduction
Thermal ablation is a minimally invasive technology, which uses thermal energy in order to irreversibly damage and destroy malignant cells.
In the past two decades, this type of treatment has been used to treat many types of tumors, including liver,kidney, lung and bone cancers, soft-tissue tumors of breast, adrenal glands, head and neck [1].
The first application of thermal ablation was described by Dupuy et al. in 2000 in three patients with lung malignancies [2]. Since then, lung ablation has been increasingly performed to treat both primary and metastatic lung disease. Indeed, based on Global Cancer Statistics 2020, lung cancer is the leading cause of cancer death, with approximately 1.8 million deaths [3], and the lungs are among the most prominent target organs for metastatic disease [4], resulting from hematogenous spread, lymphatic spread and trans-pleural diffusion.
Surgical resection represents the treatment of choice for both patients with stage I, stage II, some stage IIIA lung cancer [5] and pulmonary metastases in properly selected cases [6].
However, in old patients with multiple co-morbidities undergoing surgical resection the cardiopulmonary function may have reduced [7]. In these non-surgical candidates, thermal ablation has shown safety, efficacy and costeffectiveness in the treatment of both primary and metastatic lung tumors [8].
This therapeutic aim can be obtained using hightemperature based modality, such as radiofrequency ablation (RFA) and microwave ablation (MWA), or low-temperature based modality, such as cryoablation. Irreversible electroporation (IRE), an ablation modality that induces cells death without thermal energy usage but through pulsed electric fields, has been introduced to ablate tumors in areas previously contraindicated for thermal ablation [9].
According to these different possibilities, the pivotal role of the interventional radiologist is choosing the best procedure to perform, considering tumor and patient-related factors and obviously the available technique. Aim this review is to provide an overview about the major thermal ablation modalities, their indications and contraindications, complications, outcomes and future challenges.
Indications
As minimally invasive technique alternative to surgery, lung ablation is performed in two major conditions. The first case is represented by the patients with early-stage lung cancer who refuse to undergo surgery or are classified as “surgical unfit” due to their comorbidities. Recently, Chan et al. [10] reported that surgical resection of stage 1 non-small cell lung cancer (NSCLC) patients remains the optimal choice, whereas non-surgical stage 1A patients can benefit from the excellent results provided by ablation in terms of disease-free survival, survival cancer-specific and overall survival (OS). Thermal ablation is reported to be also effective in patients with recurrent single lesion after other local treatment (including radiotherapy [11] and surgery [12]) and in patients with a single lung [13,14]. However, it is crucial that the maximum diameter of the primary tumor be less than 3 cm and that no nodal or distant metastasis be detected [8]. The second case is represented by patients with pulmonary metastases arising from different primitive site, such as colorectal cancer [15], renal cancer [16], gastrointestinal cancer [17]. In this clinical scenario, ablation therapy should only be performed when the primary tumor is well controlled [18]. The number of metastases should be ≤ 3 for unilateral lung and ≤ 5 for bilateral lung; the maximum diameter should be ≤ 3 cm for multiple metastases and ≤ 5 cm for unilateral single metastasis, without other metastatic disease [8]. Furthermore, prognosis depends on the type of the primary tumor and the patients’ response is related to different factors: long disease-free interval (> 36 months) between the treatment of the primary tumor and the appearance of metastases, number of metastases (< 3-5), extra-thoracic locations, possibility of achieving complete ablation/resection (R0) and small size (up to 2-3 cm of larger diameter) [19].
In addition, palliative therapy is another indication for thermal ablation, in order to minimize the tumor burden, relieve symptoms caused by the tumor and improve quality of life [8].
Contraindications
Percutaneous thermal therapy has shown an excellent patient tolerance. Thus, it is difficult to identify an absolute contraindication for lung ablation, except for untreatable coagulopathies [20]. However, there are some technical limitations and particular conditions which reduce the success rate or increase the risk of failure: a life expectancy of less than 1 year [21], the treatment of bilateral lung tumors in the same session due to the relative risk of bilateral pneumothorax [22], lesions located < 1 cm from hilum, large vessel, main bronchi, trachea or esophagus [23],presence of nodal and distant metastases, an end-stage lung disease and/or respiratory failure [20].
Technique
Anesthesiologic management
These procedures may be performed either with gene-ral or local anesthesia [24,25]. A retrospective study by Hoffman et al. [26] found no difference between general and local anesthesia in terms of tumor control or complication rate. Therefore, the choice would depend essentially on the patient’s clinical conditions [24-26].
After accurate disinfection, local anesthesia is performed with the injection of Lidocaine 1-2% at the ablation applicator entry site and near the pleura; it can be associated with conscious sedation and analgesia (procedural sedation and analgesia) [27,28].
In some cases, general anesthesia may be used when the procedure is performed on a child or on a patient with low compliance, in case of time-consuming procedures, or in case of tumors located in the hilar or sub-pleural zone which are typically painful [8].
When general anesthesia is performed during cryoablations, a double-lumen endotracheal tube is used which allows the application of a continuous positive airway pressure (CPAP) in the lung to be treated, while the other lung is ventilated [29].
Despite needing a longer preparation time, general anesthesia has some advantages such as the reduction of patient movement and discomfort, thus allowing the operator to focus only on the procedure. Moreover, by using a double lumen endotracheal tube can be reduced the movement of the lung to be treated and avoided the diffusion of endobronchial hemorrhage to the contralateral lung [29]. Finally, general anesthesia may be associated with a paravertebral block in order to prevent parietal pain [30].
Pre-procedural evaluation
All the patients’ clinical data and indication to the procedure have to be evaluated before any interventional procedure by a multidisciplinary team [23,31].
It is fundamental to perform biopsy and histological analysis in order to confirm the diagnosis both in primitive and metastatic lesions, especially when the presentation is atypical [8]. Chest CT must be performed to assess the lesion (size, location, staging), ideally 2-4 weeks before the ablation procedure: it has a pivotal role in the preoperative planning as it allows the macroscopic evaluation of the tumor and its relationship with adjacent structures such as blood vessels, trachea and bronchi. Moreover, it is possible to determine the path of the ablation applicator and choose the patient’s decubitus, although in most cases the prone position is preferred since it limits respiratory movements [32].
All the patients must always be informed about the procedure benefits and possible risks and on eventual alternative treatments; for each procedure informed consent must be collected.
Each patient should undergo routine laboratory tests such as lung, hepatic and renal function and blood coagulation status, in order to exclude severe or untreatable coagulopathies.
The recommended coagulation values for percutaneous procedures are: INR < 1.5; aPTT> 1.5 times the control value if heparin is administered; platelets > 50 000 [33].
Anticoagulation and antiaggregating drugs must be suspended at least 5 days before the procedure according to the pharmacokinetic of the drug, and fasting is recommended for at least 4 hours before local anesthesia and 12 hours before general anesthesia.
Image guidance
Ablation procedures are always performed under imaging guidance, either with conventional CT, fluoroscopy CT or cone beam CT (CBCT). The most common modality is conventional CT, however new imaging techniques such as CBCT are being increasingly used as imaging guiding systems [34]. More recently, positron emission tomography (PET) has also been used as a guiding system in percutaneous treatments, thanks to its ability to detect the hypermetabolic portion of a lesion, thus allowing a more correct positioning of the ablation applicator and a more effective ablation [19].
After anesthesia, a chest CT scan is usually acquired for preprocedural planning and to evaluate the correct puncture approach, with parameters set so as to minimize irradiation of the patient and the radiology team [35]; small incision is then made with a scalpel, and the ablation probe is inserted and advanced up to the target pulmonary lesion. If the probe is not correctly positioned during this procedure, it may be repositioned under imaging guidance until it reaches a satisfactory location; after each change in position a CT scan is acquired.
In this context, CBCT is a superior imaging technique because it allows a more precise and real-time planning of the ablation applicator trajectory thanks to the combination of 3D images and fluoroscopy [35].
Moreover, thanks to 3D reconstructions, it is possible to evaluate the correct targeting of the lesion on more than one plane.
Ablation parameters such as power and time vary among different devices and the ablation time/power ratio and dimension of ablation zone is supplied by the manufacturer, in order to facilitate the decisions of the interventional radiologist who performs the procedure. The procedure success depends most importantly on the ablation margins, which should be of at least 5 or 10 mm when possible for a complete ablation [23,31,36,37].
Indeed, the main disadvantage of ablative treatments is local recurrence, which depends mainly on insufficient ablation margins, especially in large lesions: in these cases, local recurrence is usually localized in the peripheral zone of the lesion [38].
In order to include the whole tumor in the ablation area, new software’s integrated with imaging guiding systems have been recently developed, which are able to calculate the ablation area obtained depending on the set parameters (power and time), and to visualize it by fusing the calculated data with procedural images. These parameters could be modified according on lesion dimension and location and on the ablation modality: in a single location and session for tumors < 3 cm, multiple locations in a single session in case of tumors size between 3-5 cm, with multiple probes and locations for tumors > 5 cm [8].
During the ablation procedure, a parenchymal zone of increased density defined as “ground glass opacity” will appear around the target lesion as a consequence of the parenchymal damage caused by the ablation.
When this zone includes the tumor area with sufficient margins, the ablation can be considered complete, and the ablation applicator can be removed with a track ablation technique in order to avoid neoplastic seeding along the needle track.
After the ablation procedure a new chest CT is performed to exclude immediate complications (pneumothorax or hemorrhage), and to evaluate technical success and the ablation margins.
Ablation techniques
Radiofrequency ablation
Radiofrequency ablation (RFA) is the most frequent used ablation technique in solid lesions, approved by the Food and Drug Administration in 2007 for its application in lung cancer treatment [39].
RFA involves applying an electric field with frequency range of 375-500 MHz, generating heat by oscillating and colliding ions proportionally to the intensity of the radiofrequency current and causing cell death through thermocoagulation necrosis.
Currently, RFA devices used in clinical practice could be Monopolar (MP), that employs a single antenna, or Bipolar (BP) that uses dual antennas or two electrodes on the same antenna facing each other. Most electrodes in use for lung ablation operate in the MP mode.
The length and characteristics of the needle allow for modulation of the energy deposition as well as the size and shape of the ablation. Depending on the size and shape of the needle tip, a spherical ablated area, usually 2-5 cm in diameter, is generated in about 10-30 minutes [40].
The volume of RFA depends on the thermal conduction of local RFA and thermal convection between circulating blood and extracellular fluid [31]. In fact, vascular structures near the target lesion might limit the ablation effectiveness and all thermal ablation modalities because of “heat sink effect” which causes the circulating blood to dissipate thermal energy [41] (Figure 1).
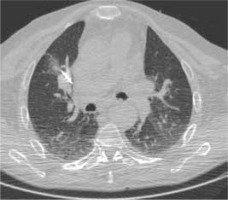
Figure 1
High resolution computed tomography (HRCT), axial plane, lungwindow. Pulmonary metastasis close to the right hilar structures, duringRFA procedure. In this case we used RFA to avoid any possible vascular,cardiac and bronchial structures lesion. No complications were observed after the procedure, that was successful on the neoplasm
Microwaves ablation
Microwave ablation (MWA) is an ablation method based on the use of electromagnetic energy, with frequency ranging between 915 MHz and 2.45 GHz.
Tissue heating is based on the agitation of polar molecules, which causes the temperature to rise rapidly to 60-150°C and induces coagulative necrosis of the tumor tissue [42,43].
MWA has greater convection than RFA, and its efficacy is not changed by the surrounding tissue impedance. Therefore, ablation may be performed at higher temperatures, also reducing the heat sink effect even for lesions close to vascular structures [44,45] (Figure 2).
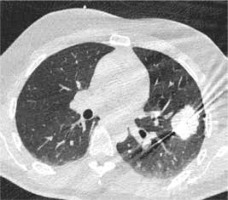
Figure 2
High resolution computed tomography (HRCT), axial plane, lungwindow. A primary lung adenocarcinoma during MWA procedure. In caseof MWA usage the time of the procedure is reduced and the efficacy of treatment is enhanced
In addition, multiple probes can be used simultaneously to achieve a larger ablation area. For successful ablation, the temperature has to be maintained homogeneously at 50-60°C for at least 5 minutes, permitting ablations up to 5 cm in a single session [19].
Cryoablation
Cryoablation (CA) consists in a combination of tissue freezing and thawing cycles, basing on the Joule-Thomsoneffect. The ablation process induces cell death through protein denaturation, membrane disruption and microvascular thrombosis [46].
The principle advantage of CA is real-time monitoring of the ablation zone with imaging techniques (CT and/or MRI), in fact the “ice ball” produced during the freezing phase is visible on CT [35]. Other advantages are that CA better preserves the collagenous architecture within the ablated tissue, to be preferred in the treatment of lesions adjacent to the bronchi, and it is featured by a low incidence of periprocedural pain [19].
Because the predominant air component of the alveolar structure may interfere with the tissue freezing, an initial 3-minute freeze-thaw cycle can be performed, which leads to fluid and hemorrhage filling of the airspaces and increases the efficacy of ablation [47].
Laser ablation
Laser ablation (LA) is a technique that causes cell death by thermal action. The most widely used laser ablation is the neodymium laser doped with yttrium aluminum garnet (Nd: YAG) with a wavelength of 1064 nm.
Specifically, the laser fiber is introduced into the lung tissue with a 21-gauge Chiba needle, the photon is adsorbed by the chromophore, generating heat and pressure on the tumor tissue.
The needle used is thinner than RFA or MWA ones, so LA generally has fewer side effects as pneumothorax [48]. If the tumor size is < 1 cm, only one laser fiber can be used, but for larger tumors it is necessary to use multiple fibers, applied in parallel or crossed positions, each of which has an output power of 3-5 W for 1800 J.
Irreversible electroporation
Irreversible electroporation (IRE) is a recent ablation method that does not use thermal energy. The working principle is to expose cells to electric pulses, inducing cell death while opening cell membrane pores. Its effects may be reversible or not basing on the applied voltage [49].
The device can deliver up to 3,000 V and 50 A, using MP or BP needle electrodes.
The size of the ablation is influenced by the length of the active tip, the duration and number of pulses, the distance between the probes, and the voltage applied.
Procedure is performed under general anesthesia with complete muscle blockage, with ECG-gating to avoid dysrhythmias during the procedure.
IRE has some advantages due to the absence of thermal effects as in other ablation techniques. Therefore, it generally has no significant side effects on connective tissue, permitting ablation of lesions also close to vulnerable structures [50,51].
Comparison between ablative techniques
Ablative techniques choice of in pulmonary lesions depends on multiple variables as lesion size, location, histopathology, and presence of patient comorbidities [52].
The first step is to choose thermal modalities (heat-based RFA and MWA or freezing-based as CA) or other techniques such as LA or non-thermal such as IRE.
Advantages of RFA include ready availability and a well-established record of safety and efficacy, but it may not be preferred for treatment of tumors with central localization, particularly near hilar structures, large vessels or airways, because of the heat sink effect. In these cases, it may be advantageous to use MWA, which can generate higher temperatures with less heat sink effect [45,53].
Other advantages of MWA over RFA include faster ablation times and preference for treating lesions near large vessels and the mediastinum and in patients with implantable cardiac devices [53].
The choice of CA usage might relate to the ability to preserve the surrounding tissues architecture. It could be advantageous in treating lesions adjacent to airways, vessels, pericardium, and bone and is characterized by minor periprocedural pain. On the other hand, CA is not recommended in patients affected by coagulopathy and bleeding tendency because of the related bleeding complications [54].
Post-procedural evaluation and follow-up
Post-procedural imaging should be acquired within minutes after the ablation to confirm procedural effectiveness and to identify any complications requiring immediate management [55].
The ablation area and the inflammatory reactions can immediately be visible in the adjacent normal parenchyma as ground-glass opacities, which is the most common finding in RFA and MWA [56]: an ablation area of 5-10 mm around the visible lesion margins suggests an effective treatment [8,19].
In contrast, the persistence of solid, nodular, and expanding areas at the site of the tumor tissue or the absence of the halo sign and/or the extension of the halo < 5 mm may suggest incomplete ablation [35] (Figures 3-5).
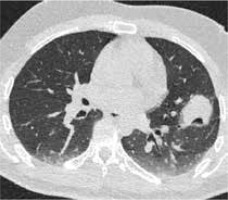
Figure 3
High resolution computed tomography(HRCT), axial plane, lung window. Pulmonary lesion immediately after RFA. Peripheral hyperdensity(“reversed halo” sign) and perilesional ground glass opacity (GGO) can be noted. The trajectoryof the needle for thermal ablation is well visible
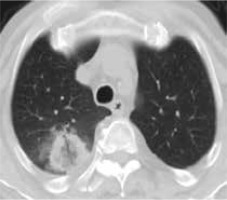
Figure 4
High resolution computed tomography(HRCT), axial plane, lung window. Pulmonary lesion the day after RFA treatment: cavitationwith hyperdense rim can be noted
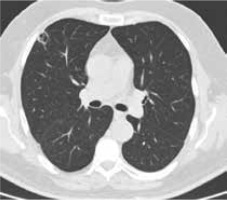
Figure 5
High resolution computed tomography(HRCT), axial plane, lung window. Pulmonary primary neoplasm treated with MWA, immediatelyafter the procedure. Intralesional cavitation and perilesional GGO – due to edema and alveolar hemorrhage – can be noted
For these reasons, follow-up of ablated tissue is gene-rally performed monthly using contrast-enhanced CT (CECT) for the first 3 months after the procedure.
Thereafter, CECT or PET/CT imaging and tumor markers tests are performed every 3 months to assess the complete ablation of the lesion and the possible appearance of new lesions/alterations.
CECT is more frequently used in clinical practice, but the potential contrast-enhanced PET-CT usage may provide a more accurate evaluation of the ablation effectiveness [8].
Complications
Percutaneous lung tumor ablation techniques are considered as relatively safe local therapies. Basing on the clinical conditions of the patients, hospitalization monitoring may vary between 24-48 h [35].
Complications may be classified into immediate (< 24 h after ablation), perioperative (24 h to 30 days after ablation), and delayed complications (> 30 days after ablation) [8].
According to a large retrospective study of 3344 patients affected by lung cancer underwent thermal ablation techniques, using data from the Nationwide Inpatient Sample (NIS) database, the most common complications occurred are pneumothorax (38.4%) and pleural effusion (4%), especially in case of RFA and MWA procedures [57]. Hemorrhage instead is more common with CA usage [58].
In clinical practice, pneumothorax is considered the most frequent immediate complication, occurred in about 30% of cases and generally asymptomatic [35,59].
Pleural effusion is reported to be occurred in 1-60% [8].
In the MWA procedure it could be more frequent the occurrence of a rare complication as bronchopleural fistula, due to higher temperatures during the procedure and a larger ablation zone [60].
Other possible complications include alveolar hemorrhage, pneumonia, pulmonary abscess, aseptic pleuritis, subcutaneous emphysema, chest fractures, nerve injury, pulmonary artery pseudoaneurysm, pulmonary infarction and death [52,53].
Complications occurred after 1 month can be consider-ed rare.
Outcome
OS is the most important indicator for clinical outcome, and OS of patients at 1, 2, 3, and 5 years is recorded. In patients who underwent palliative ablation, quantification of outcomes should be evaluated by assessment tools as quality-of-life indices and medication usage [8].
RFA is the most frequently used thermal ablation technique in primary lung cancer. A recent large meta-analysis including a sample of 1989 patients with 3025 lung cancers demonstrated a technical success rate of 96%. The same meta-analysis reported rates of recurrence and local tumor progression of 35 and 26%, respectively [52].
The prospective ACOSOG z4033 study, which enrolled inoperable stage IA NSCLC, reported OS rates of greater than 86 and 58% at 1 and 3 years, respectively [61,62].
In addition, prospective studies that demonstrated 1-, 3-, and 5-year OS rates of 97.7, 72.9, and 55.7%, respectively, indicated that RFA may be effective in treating recurrent NSCLC after surgical resection [12,63]. The American College of Chest Physicians has included RFA in its treatment recommendations for tumors < 3 cm in size [62].
MWA has 1-, 2-, 3-, and 5-year OS rates of approximately 89%, 63%, 43%, and 16%, respectively [62]. Local tumor recurrence rates are 22%, 36% and 44% at respectively 1, 2 and 3 years [53,64].
After CA the OS at 2 and 3 years is about 88% [65].
RFA, MWA, and CA have also been used in the treatment of metastatic lung lesions. In the largest reported series, in which 566 patients with 1,037 metastases were treated with RFA, the authors reported 1- and 5-year OS rates of 92.4 and 51.5%, respectively [59,63].
The results of other studies conducted with MWA and CA compare favorably with those of RFA-based studies, although the results should be interpreted with caution because smaller cohorts were treated [52,66].
In a prospective study using MWA, 80 patients with 130 metastases had a 73% complete ablation rate [53,67].
In a prospective multicenter phase 2 study of CA of lung metastases, which evaluated 40 patients with 60 metastases, measurement showed a promising 94.2% local tumor control at 12 months [53,68].
IRE is a relatively recent technique, and additional data on distant oncologic follow-up are needed. However, early clinical results of lung ablation with IRE near large vessels were disappointing, with 61% local recurrence in a multi-institutional study of 20 patients [19,69].
Limits and future perspectives
Ablation techniques have some important limitations, some of these related to the ablation only of the targeted tumor. For these reasons, pre-operative work-up with PET-CT must be very attentive for lymph nodes and/or metastases assessment for the patients’ selection [19].
In addition, the heat-sink effect and/or technical attainability of the lesion may reduce the procedural effectiveness or may result in opting for surgical treatment.
Despite these limitations, thermal ablation is more widely used for the treatment of lung neoplasms, thus new strategies are being investigating in order to tailor the treatment plan to each patient.
Radiomics is an emerging field of translational research that aims to extract high-dimensional data from clinical images [70].
Liu et al. [71] reported that changes in tissue density heterogeneity between pre- and post-ablation CT images can provide independent information to predict treatment response and survival in patients with lung malignancies.
Markich et al. [72] described that the technical, radiological, and radiomic characteristics of the target area before and after 48 hours after RFA can help discriminate nodules at risk of local progression that might benefit from a complementary local procedure.
However, current status of radiomics in lung thermal ablation is still poor and future research should be promoted.
Conclusions
Ablation of lung lesions is widely used as comprehensive lung neoplasms treatment and is one of the development directions in future, especially the image-guided percutaneous thermal ablation technology.
Thermal ablation techniques have shown safety, efficacy, and cost-effectiveness in both primary and metastatic lung tumors treatment in nonsurgical patients [73].
Currently, there are yet some limitations on thermal ablation technology in the treatment of primary and metastatic lung tumors, as the number of cases of lung lesions treated that is relatively less than that of surgery, radiotherapy, and chemotherapy [74,75] and, moreover, the presence of few clinical trials on the combination of thermal ablation and other treatment methods.
The artificial intelligence (AI) and radiomics results obtained appear very promising and will allow even more extensive and effective use of minimally invasive ablation techniques in the future.


