Introduction
Diabetic nephropathy (DN) is one of the common and serious microvascular complications of diabetes mellitus (DM). It is clinically characterized by proteinuria, edema and hypertension, and it further develops into azotemia and renal failure. 30–40% of the patients with a 15-year course of diabetes have DN, which is the leading cause of death in diabetic patients. The occurrence and development of DN is related to a variety of factors, including genetic susceptibility, glomerular sclerosis and hemodynamic changes, urinary albumin excretion, glucose toxicity [1–3] and so on.
Adiponectin (Apn) was first isolated and cloned in the mouse 3T3-L1 adipocyte cell line in 1995. It is named as adipocyte complement-associated protein 30 and is a specific protein secreted by adipocytes with a molecular mass of about 30 kD [4]. In recent years, studies have found that Apn has a certain correlation [5, 6] with obesity, type 2 DM, insulin resistance, atherosclerosis and diabetic vascular disease. It has been confirmed by animal experiments that insulin resistance is improved and blood glucose levels are reduced after injection of recombinant Apn into obese mice, suggesting that Apn has an important protective effect on diabetic damage [7].
In 2005, Degterev et al. reported a new way of cell death: Necroptosis (Nec) [8] is programmed cell death, which has morphological features similar to necrosis, while the cell death pattern is non-caspase-dependent, that is, the combination of death receptor and ligand can trigger necrotic apoptosis under the condition of caspase inhibition. Tumor necrosis factor (TNF) α combining with TNFR1 on the plasma membrane causes the TNF receptor associated death domain (TRADD) to send receptor interacting protein 1 (RIP1) signals to recruit RIP3, thereby forming a necrosome [9].
Inflammation is the main cause of Nec and increases the incidence rate of cardiovascular events in diabetic patients and the death rate of patients with renal failure [10]. Signal transduction pathways of mitogen-activated protein kinases (MAPKs) are the most critical pathways in the inflammatory transduction network [11]. This study aimed to investigate the effect of Apn on necrotic apoptosis of rat glomerular endothelial cells under high glucose (HG) and renal tissue in diabetic animal models, to clarify the possible role of Apn in the pathogenesis of DN and its signaling, and to provide a new theoretical basis for the prevention and treatment of DN.
Material and methods
Cell culture
Rat glomerular endothelial (RGE) cells were purchased from ATCC, USA, incubated in DMEM medium containing 10% fetal bovine serum (FBS) and maintained in 5% CO2 at 37°C. When the cell density reached 90%, the cells were digested and counted; 105 cells were inoculated into new culture flasks, cultured to 80% fusion in DMEM medium containing 15% FBS, and then synchronously cultured in serum-free medium for 24 h. Subsequently, they were divided into a control group (5 mmol/l glucose), HG group (30 mmol/l glucose), ApnL group (30 mmol/l glucose + 1 μg/ml Apn), ApnM group (30 mmol/l glucose + 5 μg/ml Apn), ApnH group (30 mmol/l glucose + 25 μg/ml Apn), and mannitol group (5 mmol/l glucose + 25 mmol/l mannitol), and continued to culture for 24 h.
Cell viability [12] was determined by CCK-8
RGE cells were inoculated in a 96-well culture plate. When the cells were grown to about 80% fusion, the above grouping was performed. They were incubated for different periods, then the supernatant was discarded, and washed 3 times with PBS; 90 μl of DMEM and 10 μl of CCK-8 were added to each well, incubated for 2.5 h in a 37°C incubator, and absorbance density (OD 490 nm) of the wells was recorded with a microplate reader. This experiment was repeated 5 times.
Animal model establishment
This study was approved by the Ethics Committee of Nanchang University and conducted in accordance with the Declaration of Helsinki and the Guide for the Care and Use of Laboratory Animals. Thirty male, standard, clean, healthy Sprague-Dawley (SD) rats were provided by the Experimental Animal Center.
Diabetes model preparation: STZ was dissolved in citrate buffer (pH = 4.5) in the weight ratio of 60 mg/kg; under fasting conditions, intraperitoneal injection was performed; the normal control group was only injected with an equal amount of citrate buffer. The blood glucose level was measured at 72 h, and ≥ 16. 7 mmol/l indicated that the diabetes animal model was successfully established. Among the 30 rats, in 28 the blood sugar reached the standard, with a success rate of 93.3%.
DN model preparation: 24 h urine protein was measured at 4 weeks, and ≥ 30 mg/day indicated that the DN models were successfully established. Among the 28 rats, 20 reached the standard, with a success rate of 71.4%. The successful DN animal model was randomly divided into a model group (n = 10) and a DN/Apn group (n = 10), and 2 mg/kg/day Apn was injected into the tail vein of the rat models in the DN/Apn group for 6 weeks. Eight SD rats with matched age and body weight were finally selected as the normal control group. At the end of the study, the animals were injected with isoflurane overdose and subjected to cervical dislocation to sacrifice the animals.
Collection and testing of specimens
In 6 weeks after the Apn administration, the rats were placed in a metabolic cage, and urine was collected in 24 h. After filtration, the rats’ urine was stored at –70°C for urine protein quantification. Serum creatinine and urine creatinine were measured using an automatic biochemical analyzer, and the creatinine clearance rate (Ccr) was calculated using the following equation: Ccr = urine creatinine × urine volume/serum creatinine × 1440/body weight. Then, the rats were weighed, anesthetized by intraperitoneal injection with sodium pentobarbital at a weight ratio of 45 mg/kg, and the left kidneys of the rats were quickly weighed. After the right kidney was lavaged with normal saline, some right kidney tissues were taken and fixed with 10% neutral formaldehyde, and embedded in paraffin for immunohistochemistry and hematoxylin-eosin (HE) staining. Some kidney tissues were taken separately for western blot analysis.
Renal pathologic examination
Renal tissue was fixed with 10% neutral formaldehyde, embedded in paraffin, sliced in sections 4 μm thick, and stained with HE in order to observe the degree of mesangial matrix hyperplasia as well as glomerular, renal tubule and renal interstitial lesions. Glomerular hypertrophy was confirmed by measuring the mean glomerular diameter of 20 glomeruli. Renal tissue sections were routinely dewaxed and dehydrated, incubated in 3% hydrogen peroxide for 10 min at room temperature to eliminate endogenous peroxidase, and blocked with serum for 10 min, then rabbit anti-rat RIP1 and PIP3 antibody were added (1 : 200, overnight at 4°C), and washed with phosphate buffer saline (PBS) twice, 3 min each time. On the next day, biotin-labeled secondary antibody was added to each section, incubated at 37°C for 30 min, and then washed twice with PBS, 3 min each time. Next, HRP-labeled streptavidin was added to each section, incubated at 37°C for 30 min, and then washed twice with PBS, 3 min each time. After they were developed with diaminobenzidine (DAB) for 5–20 min at room temperature, they were slightly counterstained with hematoxylin-eosin.
Western blotting
Cells or kidney tissues were lysed on ice for 30 min with cell lysis solution (containing protease inhibitor cocktail). They were centrifuged at 12 000 × g for 15 min at 4°C, and then the supernatant was taken. Protein quantification was performed by the bicinchoninic acid (BCA) method. A 50 μg sample was loaded in each group, and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 5% concentrated gel and 8% or 10% separating gel to separate the proteins (RIP1, PIP3, p-p38MAPK and t-p38MAPK). These proteins were transferred to a polyvinylidene fluoride (PVDF) membrane at 220 mA electric current for 1 h; then they were blocked for 1 h at room temperature; after that they were washed 3 times with tris buffered saline Tween (TBST), and the corresponding primary antibodies were incubated overnight at 4°C. The next day, they were incubated with corresponding secondary antibody for 1 h at room temperature; then, they were incubated with an enhanced chemiluminescence solution for 2 min, and quantified using Alpha-Ease software version 2200 [13].
Results
Apn protected HG-induced Nec
Figure 1 A shows that treatment of RGE cells with HG (30 mmol/l) for 24 h can induce significant cytotoxicity and thus reduce cell viability; compared with the normal control group, the difference is statistically significant. The cell viability was increased by co-treatment of the cells with 1, 5 or 25 μg/ml Apn and HG for 24 h, which was statistically significant (p < 0.05); the inhibitory effect of 25 μg/ml Apn on cytotoxicity was most pronounced.
Figure 1
Apn protects HG-induced Nec. A – Cell viability was assayed by CCK-8 assay. Treatment of RGE cells with HG (30 mmol/l) for 24 h can cause significant cytotoxicity. Cell viability was promoted by co-treatment of the cells with 1, 5 or 25 μg/ml Apn. B – The expression of RIP1 and RIP3 in cells were examined by immunofluorescence. C – The expression of RIP1, RIP3 and p-p38MAPK were assayed by western blot. The expression levels of RIP1, RIP3 and p-p38MAPK in HG-treated cells were significantly increased. Co-treatment of cells with 1, 5 or 25 μg/ml Apn could reduce the expression of RIP1, RIP3 and p-p38MAPK
*p < 0.05 and **p < 0.01 vs. Control; #p < 0.05 and ##p < 0.01 vs. HG.
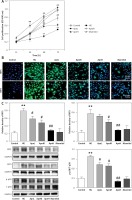
We tested the expression of RIP1 and RIP3 in cells by further application of immunofluorescence and western blot. The expression levels of RIP1 and RIP3 in cells treated with HG for 24 h were significantly increased. Co-treatment of cells with 1, 5 or 25 μg/ml Apn and HG for 24 h could reduce the expression of RIP1 and RIP3. The differences were statistically significant (Figures 1 B, C).
The expression of p-p38MAPK was also significantly increased by treating the cells with HG for 24 h; compared with the normal control group, the difference was statistically significant (p < 0.01). The expression of p-p38MAPK was significantly decreased by co-treatment of cells with 1, 5 or 25 μg/ml Apn and HG for 24 h (p < 0.01, Figure 1 C).
Apn improved DN
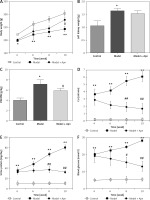
After 10 weeks, STZ rats clearly showed symptoms of polydipsia, polyuria and polyphagia, and their body weights were significantly reduced. In addition to the death of 3 rats in the model group, the animals in other groups were in a good condition. The body weight, left kidney weight, left kidney weight/body weight (KW/BW, g/kg), Ccr, urine protein and blood glucose for each group of rats are shown in Figure 2. Compared with the rats in the control group, the body weights of the rats in the model group were significantly reduced from the 4th week; the body weights of the rats increased from the 6th week after Apn intervention (p < 0.05). KW/BW (g/kg) of the rats in the model group was significantly increased, and decreased after Apn intervention. Ccr, 24 h urine protein and blood glucose levels of the rats in the model group increased significantly from the 4th week (p < 0.01), while they significantly decreased from the 4th week after Apn intervention (p < 0.01).
Figure 2
Apn improves DN animal models (n = 8). Compared with the rats in the control group, the body weights of the rats in the model group were significantly reduced from the 4th week, and were increased from the 6th week after Apn intervention. The left kidney weight and KW/BW of the rats in the model group were significantly increased and KW/BW significantly decreased after Apn intervention. Ccr, 24 h urine protein and blood glucose from the rats in the model group increased significantly from the 4th week, and significantly decreased from the 4th week after Apn intervention
*p < 0.05 and **p < 0.01 vs. Control; #p < 0.05 and ##p < 0.01 vs. Model.
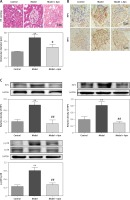
Apn reduced the expression of RIP1, RIP3 and p-p38MAPK
HE staining showed that, for the rats with DN, their glomerular diameters were significantly increased (greater than the rats in the control group), their glomerular mesangial matrix was proliferated, focal lymphocytes and monocyte infiltration were observed in the interstitial region, and some of the epithelial cells of the renal tubules began to develop vacuolar degeneration and even partially fall off. Compared with the rats in the model group, the glomerular capillary loops of the rats in the Apn group were well opened, the mesangial matrix was slightly proliferated, a small amount of inflammatory cells infiltrated, and some renal tubular epithelial cells began to repair (Figure 3 A). The results of immunohistochemistry of RIP1 and PIP3 in renal tissues showed that RIP1 and PIP3 were mainly expressed in renal tubules of the kidney tissues of DN rats (Figure 3 B). Western blot results showed that the expression levels of RIP1 and PIP3 proteins of the rats in the model group were significantly increased, and were significantly decreased after Apn intervention (Figure 3 C). Moreover, the activation of p-p38MAPK in renal tissue of DN rats was also significantly increased, and was significantly decreased after Apn intervention (Figure 3 C).
Figure 3
Apn reduced the expression of RIP1, RIP3 and p-p38MAPK (n = 8). A – HE staining. The glomerular diameter was significantly increased in the model group, and was decreased after Apn intervention. B – Immunohistochemistry of RIP1 and PIP3 in renal tissues. C – Western blot assay. The expression levels of RIP1, PIP3 and p-p38MAPK for the rats in the model group were significantly increased, while they were significantly decreased after Apn intervention
**p < 0.01 vs. Control; #p < 0.05 and ##p < 0.01 vs. Model.
Discussion
DN is one of the common chronic complications of diabetes, and Nec plays an important role in its pathogenesis [14]. It has been confirmed that it involves the participation of RIP1 and RIP3. RIP1 is the first confirmed important molecule that regulates programmed cell necrosis under the conditions in which caspase is inhibited. RIP1, as an upstream kinase, can regulate RIP3-dependent programmed necrosis, and induce formation [15] of the RIP1-RIP3 necrosome under the action of TNF-α.
According to modern endocrinology, adipose tissue is not only the main energy storage organ, but also an endocrine organ of the body, and meanwhile it can also secrete a variety of biologically active factors. Previous studies found that Apn has the functions of improving insulin sensitivity, lowering blood glucose and blood lipid, resisting atherosclerosis, inhibiting inflammatory reactions, resisting immunity and protecting microvessels [16, 17]. The results of this experiment showed that the expression of RIP1 and RIP3 in RGE cells was significantly decreased under HG stimulation after Apn intervention in a concentration-dependent manner, indicating that Apn might reduce the inflammatory response and damage of RGE cells and promote cell repair by inhibiting the Nec pathway. The p38MAPK pathway is one of the important members of the MAPK family, can be activated by various stimuli such as inflammation, ischemia, hypoxia, chemical damage, etc., and can participate in the regulation of pathological and physiological processes [18] such as cell growth, differentiation and apoptosis. Our study suggests that the p38MAPK pathway can mediate HG-induced cell damage. Apn can significantly inhibit the up-regulation of p-p38MAPK expression caused by HG, suggesting that Apn can inhibit Nec and improve DN by inhibiting the p38MAPK signaling pathway.
STZ specifically destroys islet β-cells and different doses of STZ induce different degrees of islet damage. A large dose of STZ (60 mg/kg) induces diabetes similar to the acute phase of human type I diabetes [19]. In the present study, we found that proteinuria in rats increased significantly at 4 weeks, and increases progressively with time. In animal models, we found that Apn significantly improved DN including increasing the body weight and KW/BW and decreasing Ccr, 24 h urine protein and blood glucose. Also, Apn reduced the expression of RIP1, RIP3 and p-p38MAPK and decreased the expression of RIP1 and RIP3.
In conclusion, Apn can alleviate the inflammatory response and damage of DN by inhibiting the Nec pathway via the p38MAPK pathway.


