Introduction
Malignant tumors have recently become one of the main diseases threatening the survival of human beings. In China, there are more than 1.6 million new cancer patients each year and it has become a major cause of death [1]. The main therapeutic function of antineoplastic drugs is to prolong and improve the quality of life of cancer patients; in this respect, traditional Chinese medicine has advantages. Buckwheat has the benefit of reducing blood pressure, reducing blood glucose and reducing lipids [2–5], and also has antineoplastic effects [6–9]. Buckwheat polysaccharide (BP) is a kind of polysaccharide extracted from the flowers and leaves of buckwheat. An earlier study indicated that it was able to increase the immunologic function of experimental animals. The current study evaluated the adjuvant therapeutic effect of BP on S180 sarcoma mice.
Material and methods
Cell culture and animal model
S180 tumor cells were provided by the School of Preventive Medicine, North China Coal Medical University and maintained in a RPMI-1640 medium supplemented with 10% fetal bovine serum. The culture transfer cycle is 6 days. The S180 tumor-bearing model was established as previously described [10]. S180 mouse sarcoma cells were briefly diluted to a final concentration of 1 × 106/ml and inoculated subcutaneously into the right armpit or the abdominal cavity of healthy Kunming mice. The male-to-female ratio was 1 : 1. The weight was 19 ±3 g. The mice were provided by the Institute of Laboratory Animals Science, CAMS & PUMC. The qualified certificate number is No. SCXK (J) 2005-0013. The mice were fed in the barrier environment of the animal center of North China Coal Medical University. All animal experimental procedures were approved by the Animal Care and Use Committee of North China Coal Medical University and performed in accordance with the Guide for the Care and Use of Laboratory Animals [11].
Main instruments
Electronic balance (Shanghai Second Balance Instrument Plant), automatic dual pure water distiller SZ-93 (Sichuan Yaxi Machinery Co., Ltd.), carbon dioxide incubator (Forma Scientific Company), inverted phase-contrast microscope UFX-ILA (OLYMPUS), clean bench (Forma Scientific Company), TGL-16G desk centrifuge (Shanghai Anting Scientific Instrument Plant) and flow cytometer (Becton-Dickinson Company).
Drugs and reagents
Buckwheat polysaccharide: extracted by the pharmacologic teaching and research office of the North China Coal Medical University. Cyclophosphamide (CTX, Shang Hualian Pharmaceutical Co., Ltd., batch No.: 080612), PI, RNA enzyme and cell permeability agent (Beijing Solarbio Life Sciences Co., Ltd.).
Extraction method of buckwheat polysaccharide
Distilled water (5000 ml) was poured into the flowers and leaves of buckwheat (500 g) (volume/weight = 10 : 1). Then, it was boiled at 95°C twice (2 h and 1 h, respectively), and then filtered. After that, the filtrate was concentrated to 1/3 of the original solution. 95% ethanol was added and the crude polysaccharide was produced after sedimentation. Distilled water was added to the crude polysaccharide. 3% trichloroacetic acid was added at a ratio of 1 : 1 to remove protein. It was then left to stand for 24 h to obtain the liquid supernatant by centrifugation. Again, 95% ethanol was added for precipitation. Holosaccharide was then obtained after centrifugation and sediment drying.
Suppression of buckwheat polysaccharide on S180 sarcoma mice
Ascitic fluid was taken from S180 tumor-bearing mice that received tumor cell transplantation and culture for 6 days. The ascitic fluid was washed with normal saline twice, counted, and the number of cells was adjusted to 1 × 108/ml. The fluid was subcutaneously injected into the mice’s right axilla in accordance with the dose of 0.2 ml per mouse. The mice were randomly divided into six groups after inoculation: a high-dose BP group (BP-H, 400 mg/kg), a medium-concentration group (BP-M, 200 mg/kg), a low-concentration group (BP-L, 100 mg/kg), a CTX (150 µg/ml) positive control group, a BP-M+CTX (200 mg/kg + 150 µg/ml) group and a model control group. The number of mice in each group was 24. There was no significant difference in the baseline body mass among the groups. After inoculation, the BP was re-dissolved in 0.9% saline to the designated doses and intragastrically administered to the mice. Intraperitoneal injections were carried out for the mice in the CTX group. The mice in the model group received a p.o. administration of 0.9% saline (25 ml/kg). After successive administration for 14 days, the mice were weighed on the second day of the final administration, and executed by cervical vertebra dislocation. The tumor of each mouse was removed. The tumors were weighed, after the blood had been blotted up by filter paper, to calculate the anti-tumor rate.
Anti-tumor rate/% = (1 – average tumor mass of the experimental groups/average tumor mass of the control groups) × 100%.
Effects of buckwheat polysaccharide on the thymus index and spleen index of S180 tumor-bearing mice
The thymus and spleen of the above mice were taken and weighed, after the blood on the surface was blotted up by filter paper, to calculate the thymus index and spleen index.
Thymus (spleen) index = thymus (spleen) mass (mg)/weight (g) × 100%.
Effects of BP on the lifespan and survival rate of S180 mice with solid tumor(s)
Mouse tumor inoculation, administration and grouping were the same as above. There were 24 mice in each group. The first day was the same day of administration. The experimental time was 25 days. The general conditions and time of death of the tumor-bearing mice were recorded to calculate the average lifetime and survival rate after 25 days.
Lifetime = (the sum of the survival time of the animals in the experimental group/the number of the animals in the experimental group) × 100%.
Survival rate after 25 days = (the number of animals that survived after 25 days/the number of the animals in the experimental group) × 100%.
Effects on lifespan and survival rate of S180 ascitic tumor mice
Mouse tumor inoculation, administration and grouping are the same as above. There were 24 mice in each group. The first day was the same day of administration. The experimental time was 25 days. The general conditions and time of death of the tumor-bearing mice were recorded to calculate the average lifetime and survival rate after 25 days.
Average lifespan = (the sum of the survival time of the animals in the experimental group/the number of the animals in the experimental group) × 100%.
Survival rate after 25 days = (the number of animals that survived after 25 days/the number of the animals in the experimental group) × 100%.
Cell apoptosis detection via flow cytometry
Ascitic fluid from the tumor cells of the S180 mice that had been inoculated for three days was drawn, and the fluid was washed with Hanks solution twice to adjust the cell concentration to 106/ml. The culture medium with cells was placed on a 24-well culture plate. These cells were divided into 6 groups with 6 duplicate wells, each well containing 0.6 mL of solution. Different drugs were then added to the culture wells in each group: the model group (with appropriate culture medium added); the high, medium and lower concentration groups (the BP solution concentration in the culture solution was 150 µg/ml and 75 µg/ml, respectively); the CTX group (the final concentration of CTX in the culture solution was 150 µg/ml), the CTX (150 µg/ml) + BP-M (150 µg/ml) group. It was ensured that the final volume of the groups was the same. After 24 h, the cells were collected into sterile centrifuge tubes. The liquid supernatant was removed by centrifugation twice. Then, 70% cold ethanol was added and the solution was placed in the refrigerator at –20°C. During analysis, the stored cell sap was taken to be washed with phosphate buffer saline (PBS) twice. After that, the liquid supernatant was removed by centrifugation, and the BPS solution was re-suspended. The prepared propidium iodide (PI) dying liquor and RNA enzyme were added to produce a reaction by keeping it in the dark for 15 min. The solution was filtered by a 400-mesh net and then analyzed by computer.
Results
Effects of buckwheat polysaccharide on lifespan and survival rate of S180 mice with solid tumor(s)
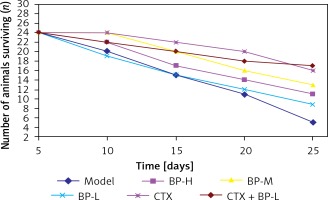
After the mice were inoculated with S180 sarcoma cells, the right fore armpits of the mice were enlarged after about 6 days, and the right fore armpit of the mice grew larger with time. In the model control group and the CTX group, the mice gradually reduced their activity and diet. The luster of the mice’s fur became dull and the mice were slow to respond. All the above conditions gradually became more serious. These conditions were rather more severe in the CTX group. The activity, diet, fur luster and mental conditions of the mice in the group with different BP doses were better than those of the mice in the CTX group and the model control group. From the 9th day, the mice in the model group and the CTX group began to die. The results during the 25-day observation period are shown in Figure 1 and Table I.
Effects of buckwheat polysaccharide on lifespan and survival rate of S180 ascitic tumor mice
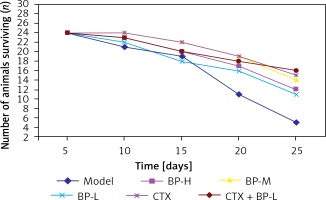
For the mice with S180 ascitic fluid sarcoma strains inoculated in the abdominal cavity, their abdomen began to enlarge on the 5th day, and the abdomen girth and size increased with time. The activity, diet and mental conditions of the mice in the group with different BP doses were better than those of the mice in the CTX group and the model group. From the 6th day, the mice in the CTX group began to die. The results during the 25-day observation period are shown in Figure 2 and Table II.
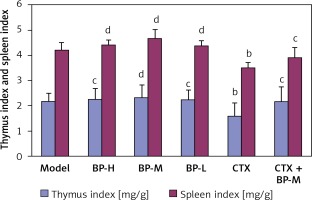
Effects of buckwheat polysaccharide on the thymus and spleen indexes of S180 sarcoma mice
The thymus and spleen indexes of the mice in the group with different doses were higher than those of the mice in the model group; however, the differences were not statistically significant. The thymus index and spleen index of the mice in the CTX group were significantly reduced, while BP-M successfully controlled the lowering of the thymus index and spleen index caused by CTX (Figure 3).
Observation of BP for the inhibition rate of mice S180 sarcoma
As for the weights of the tumors in each group, the tumors in the model group were the most severe. The weight of the tumors in the CTX and CTX + BP groups was significantly lower than that in the model group (p < 0.01). High, medium and low doses of BP had the ability to suppress the growth of mouse S180 sarcoma. However, the difference compared with the model group was not statistically significant (p > 0.05). The difference in weight of the tumors in the CTX and CTX + BP-M groups was not statistically significant. The anti-tumor rate was significantly higher than in the BP-H, BP-M and BP-L groups. The detailed results are shown in Table III.
Table III
Effect of buckwheat polysaccharide (BP) on inhibiting S180 tumor in SA mice (x ± s, n = 24)
| Group | Dose [mg/kg] | Tumor weight [g] | Inhibition rate (%) |
|---|---|---|---|
| Model | 3.28 ±0.46d | ||
| BP-H | 400 | 3.00 ±0.40d | 9.04 ±1.55 |
| BP-M | 200 | 2.98 ±0.45d | 9.27 ±2.21 |
| BP-L | 100 | 3.04 ±0.36d | 7.37 ±1.71 |
| CTX | 3 | 1.91 ±0.31b | 42.67 ±8.61 |
| CTX + BP-M | 3 + 200 | 1.69 ±0.38b | 46.67 ±9.71 |
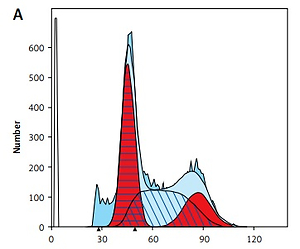
Cell cycle detection via flow cytometry
In the G0/G1 phase, the cells of the tumor cells in the group with different doses of BP increased, with the BP-M group having the most obvious increase. However, the difference compared with the model group was not statistically significant (p > 0.05). It indicated that BP was able to arrest the cycle of the tumor cells in the G0/G1 phase. The cell content of the tumor cells in the CTX group greatly increased in the G2/M phase compared with the model group (p < 0.01), while the content in the G0/G1 phase was significantly lower than the model group (p < 0.05), which indicated that the drug mainly arrested the tumor cells in the G2/M phase. Details are shown in Figure 4 and Table IV.
Table IV
Effect of buckwheat polysaccharide (BP) on cell cycle of S180 ascites tumor mice (x ± s)
| Group | Dose [μg/ml] | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|---|
| Model | – | 44.16 ±1.03 | 44.18 ±2.70 | 11.66 ±3.06 |
| BP-H | 300 | 45.19 ±2.13d | 42.11 ±3.27 | 12.59 ±1.16d |
| BP-M | 150 | 47.30 ±4.70d | 40.88 ±4.54 | 11.84 ±0.68d |
| BP-L | 75 | 46.26 ±4.67d | 42.47 ±3.98 | 11.27 ±1.36d |
| CTX | 150 | 39.71 ±2.49a | 42.72 ±1.12 | 17.57 ±2.48b |
| CTX + BP-M | 150 + 150 | 43.71 ±3.18c | 42.93 ±1.01 | 13.37 ±2.85d |
Discussion
The types of drugs available for the treatment of malignant tumors are varied [12, 13]. Chemotherapeutic drugs such as CTX, etc., can suppress the growth of tumors, but the toxic side effects remarkably lower the quality of the patients’ lives. Traditional Chinese medicine has gradually become a research hotspot for its advantages of high efficiency, low toxicity, and overall adjustment [14, 15]. The study of immunocompetence polysaccharide in the aspects of promoting and adjusting immunologic function has progressed greatly, and the study of its mechanism of action has been deepened at the molecular and genetic levels [16–22]. At present, the mechanism for polysaccharide antineoplastic study has two main aspects: one is that the polysaccharide directly kills tumor cells; the second is that the polysaccharide achieves the indirect antineoplastic effect by activating or enhancing immunologic function [23]. The documents related to this study indicated that, at the same time as enhancing the immunity, this polysaccharide has no direct toxic side effects on normal cells. Therefore, this polysaccharide has been recognized as a very important biological effect regulator [24–26].
The results of this experiment indicate that BP had a certain suppressing effect on mouse S180 sarcoma; however, the difference was not statistically significan. BP was able to improve the lifespan, survival rate and survival quality of S180 sarcoma mice. Its application alone was able to improve the thymus and spleen index of tumor mice, while its combined application with CTX was able to improve the suppressing effects of CTX on the thymus index and spleen index of tumor mice. A flow cytometer was used to observe the effects of BP on tumor cell cycle. It was found that BP was able to arrest the tumor cell cycle in the G0/G1 phase to prevent cell transformation. The experimental results indicated that BP did not have obvious antineoplastic effects, but it could prolong the lifespan of the tumor mice and improve the survival quality. In addition, it was able to prevent the suppressing and toxic reaction of CTX on immunologic function, which functions as adjuvant therapy for tumors.
There are some limitations to the present study. Firstly, the effect of BP in adjuvant therapy was solely investigated in S180 xenografts. Further studies on different tumor-bearing models will be needed to confirm the beneficial effect of BP in tumor progression. Secondly, we did not explore the molecular mechanisms of BP in extending the lifetime of tumor-bearing mice. Further investigation will be required to identify the potential regulatory targets of BP in tumor progression.
In conclusion, our results showed that BP significantly extended the lifespan, increased the survival rate and reduced the toxic effect of CTX in S180 tumor-bearing mice. The adjuvant therapeutic function of BP deserves further investigation to determine its role in the treatment of cancer.