Introduction
Excessive consumption of fat and fructose leads to obesity due to lipid accumulation [1, 2]. The greater calorie intake than energy expenditure leads to accumulation of lipids in fat tissue [3]. Excessive accumulation of lipids, especially triglycerides, can cause adipocyte hypertrophy and hyperplasia [4]. Hypertrophy adipocytes are damaged and die [5] causing pro-inflammatory cytokine production by fat tissue [6]. Pro-inflammatory cytokines on fat tissue cause the infiltration of immunocompetent cells into the fat tissue, thereby triggering excessive production of pro-inflammatory cytokines [7, 8].
Toll-like receptor (TLR) is an inflammatory mediator that can be activated by lipopolysaccharide (LPS) and other ligands, such as danger-associated molecular pattern (DAMP) [9]. Elevating the DAMP production causes increased expression of TLR3 and TLR4, which have an inflammation mediator function [10].
Soybean (Glycine max) has the potential to eliminate the negative effect of overweight [11, 12]. Soybean is a source of isoflavone, which in elicited soybean extract was higher than unelicited soybean extract. A recent study suggest that the combination of microorganism and light could enhance the isoflavone profile compared to microorganism alone [13]. Secondary metabolites of elicited soybean are mainly genistein, daidzein, and glyceollin [14, 15]. Genistein can cause lipolysis and prevent the adipogenesis process, thus inhibiting the formation of adipocytes [16, 17]. Glyceollin on soybean can suppress inflammation by inhibiting the activation of NF-kB, thus suppressing the increased production of pro-inflammatory cytokines [18]. Daidzein has a role in reducing plasma cholesterol levels by increasing the cholesterol metabolism in hepatocytes [19]. Based on that fact, we assumed that elicited soybean extract could be an alternative therapeutic in the future to attenuate inflammation caused by a high-fat diet.
Material and methods
Elicited soybean extract (ESE) preparation
Soybean (Glycine max) var. Anjasmoro was purchased from the Indonesian Legumes Tuber Crops Research Institute (ILETRI), Malang Regency. Soybeans were soaked in 70% alcohol for 10 minutes at room temperature then washed with sterile distilled water four times [20].
Soybeans were soaked in distilled water and placed in a dark room for a day. Soybeans were grown and inoculated with 1 × 107 S. cerevisiae (7.5 ml of suspension/100 γ soybean) in a sterile plastic wrapped box. The box was stored at room temperature with light exposure using a bulb lamp [13]. The extraction method was done as previously described [21].
Soybeans were harvested at day 3 and washed three times using distilled water. 100 g of soybeans were weighed and made into a paste with 70% ethanol (300 ml) using a blender. The blended soybean was incubated at 50°C for one hour. The soybean was centrifuged for 10 minutes at 4500 rpm at room temperature. The supernatant was filtered with a 0.45 µm sterile filter (BD Falcon). The alcohol content of the filtrate was evaporated with a rotary evaporator and then freeze-dried. The elicited soybean extract was then stored at –20°C until use.
Chemical
Simvastatin 10 mg was purchased from Dexa Medica (Tangerang, Indonesia). PE-conjugated rat anti-mouse TLR4, PE/Cy5-conjugated rat anti-mouse TLR3, PE-conjugated rat anti-mouse TNF-α, and PE-Cy5 conjugated anti-mouse IFN-γ were purchased from BioLegend® (San Diego, CA).
Animals and experimental design
A total of 28 normal neonatal Balb/C female mice, three weeks old, and weighing 10-12 g were used as a model. Mice were obtained from the Integrated Research and Testing Laboratory-Unit IV, Gadjah Mada University. The experimental procedures were approved by Brawijaya University Research Ethics Committee (Animal Care and Use Committee); serial no. 647-KEP-UB.
This study used four replications in seven treatments: (1) normal diet group (negative control); (2) HFFD group (positive control); (3) normal diet group with normal dose of ESE; (4) HFFD group with simvastatin 2.8 mg/kg BW treatment; (5) HFFD group with low dose of ESE treatment (78 mg/kg BW); (6) HFFD group with normal dose (104 mg/kg BW) of ESE treatment; and (7) HFFD group with high dose of ESE treatment (130 mg/kg BW). Simvastatin and ESE were administered orally once a day for four weeks. The normal dose is the conversion of the dose applied in humans to an animal model, based on FDA (Food and Drug Administration).
Fructose and high-fat diet
The normal (ND) diet group was fed with normal feed and mineral water ad libitum. The ND was provided by PT Galaxy Science, Jember, Indonesia and consisted of 67.27% carbohydrate, 12.73% protein, and 5.33% fat. The HFFD group were fed with high fat and fructose feed, which was modified from previous research [22]. The HFFD feed consisted of 53.46% carbohydrate, 8.57% protein, and 21.06% fat. 10% fructose solution mixed with mineral water. The diet was given for over 20 weeks [23].
Isolation of lymphocytes
Mice were dissected after four-week treatments. The rats’ spleens were isolated and crushed clockwise in PBS. Lymphocyte homogenates were moved into a new propylene tube, 10 ml of PBS was added, and then they were centrifuged at 2500 rpm, 10°C, for five minutes. The pellet was resuspended in 1 ml of PBS, and then 5 µl of it was transferred into 1.5 ml microtubes.
Antibody staining
Pellet solution was stained with extracellular and intracellular antibodies. Fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD4 and phycoerythrin (PE)-conjugated anti-mouse CD8 were used as extracellular antibodies. For extracellular staining, the pellet was resuspended in 50 ml of antibodies in sterile PBS. Antibodies for intracellular staining were PE-Cy5-conjugated anti-mouse IFN-γ, PE-conjugated anti-mouse TNF-α, PE-conjugated rat anti-mouse TLR3, and PE/Cy5-conjugated rat anti-mouse TLR4. Intracellular antibody staining is done by adding 50 ml cytofix-cytosperm into pellets and then incubating for 20 min at 4°C. After that, the solution was added to 500 ml of washperm and centrifuged at 2500 rpm, 4°C, for five minutes. Furthermore, pellet and antibody solutions were resuspended in 50 ml of sterile PBS.
Flow cytometry analysis
Cells were transferred into the cuvette and mounted on the nozzle of flow cytometer (BD FACS CaliburTM). BD Cell Quest Pro softwareTM was set on the computer after that and then connected with the flow cytometer (acquiring mode). Flow cytometry analysis was conducted to determine the relative number of CD4+TLR3+ T, CD4+TLR4+ T, CD4+TNF-α+ T, and CD4+IFN-γ+ T cells.
Results
Suppression of TLR3 and TLR4 activation
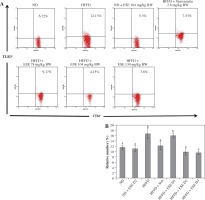
TLR is a trans-membrane protein that acts as inflammation mediator. ESE treatment in HFFD mice model is capable of inhibiting the activation of TLR3+ in normal and high doses, compared to an untreated HFFD mouse model (Fig. 1A). TLR3+ can be activated when it binds to a ligand such as DAMP, like an mRNA of dying adipocytes under obesity conditions [11]. The relative number of CD4+ TLR3+ T cells declines after ESE treatment at normal and high doses, which is related to the inhibition of TLR3 activation (Fig. 1B).
Fig. 1
Expression of TLR3 after elicited soybean extract (ESE) administration. A) Flow cytometry analysis showed the expression of TLR3 on CD4 T cells was decreased after administration with normal and high doses of ESE. B) The graph is a calculation of a relative number of CD4+ T cells expressing TLR3+ T cells on spleen cells after ESE treatment for four weeks. Data are mean ±SD values of each group, p ≤ 0.05. The difference of abjad indicated a significant difference by Duncan test. D1 – ES 78 mg/kg BW (low dose), D2 – ESE 104 mg/kg BW (normal dose), D3 – EKE 130 mg/kg BW (high dose), ND – normal diet, HFFD – high-fat and -fructose diet, ESE – elicited soybean extract, Sim – simvastatin 2.8 mg/kg BW
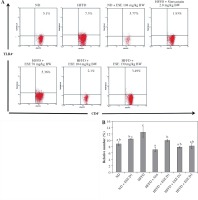
The results showed that ESE treatment is able to suppress TLR4 activation (Fig. 2A). All of the ESE doses can reduce the relative number of CD4+TLR4+ T cells compared to untreated HFFD mice (p < 0.05) (Fig. 2B). The normal and high dose of ESE can decrease the relative number of TLR3+ and TLR4+ cells significantly (p < 0.05) compared to the HFFD mouse model without treatment, while the low dose of ESE was only able to suppress the activation of TLR3+.
Fig. 2
Expression of TLR4 activation after elicited soybean extract (ESE) administration. A) Flow cytometry analysis showed the expression of TLR4 on CD4 T cells was decreased after administration with all doses of ESE. B) The graph is a calculation of a relative number of CD4+ T cells expressing TLR4+ T cells on spleen cells after ESE treatment for four weeks. Data are mean ±SD values of each group p ≤ 0.05. The difference of abjad indicated a significant difference by Duncan test. D1 – ES 78 mg/kg BW (low dose), D2 – ESE 104 mg/kg BW (normal dose), D3 – EKE 130 mg/kg BW (high dose), ND – normal diet, HFFD – high-fat and -fructose diet, ESE – elicited soybean extract, Sim – simvastatin 2.8 mg/kg BW
Inhibiting pro-inflammation cytokines
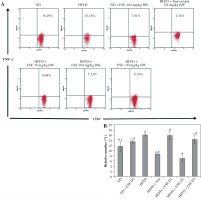
The elevation of TLR3 and TLR4 activity in the HFFD mouse model was also followed by the increase of pro-inflammatory cytokines number as TNF-α and IFN-γ. Normal-diet mice had the relative number of T CD4+TNF-α+ at 11.63% of the T CD4 population (Fig. 3A). The average number of T CD4+ TNF-α+ in the HFFD mice model was higher than in the normal mice, at about 16.15% of the T CD4 population of the cell. ANOVA analysis (Fig. 3B) indicated that normal-dose ESE administration can significantly reduce the expression of TNF-α compared to the untreated HFFD mouse model (p < 0.05). Simvastatin also reduced TNF-α expression in HFFD mice.
Fig. 3
The expression of TNF-α after elicited soybean extract (ESE) administration. A) Flow cytometry analysis showed the expression of TNF-α on CD4 T cells was decreased after administration with the normal dose of ESE. B) The graph is a calculation of a relative number of CD4+ T cells expressing TNF-α on spleen cells after ESE treatment for four weeks. Data are mean ±SD values of each group p ≤ 0.05. The difference of abjad indicated a significant difference by Duncan test. D1 – ES 78 mg/kg BW (low dose), D2 – ESE 104 mg/kg BW (normal dose), D3 – ESE 130 mg/kg BW (high dose), ND – normal diet, HFFD – high-fat and -fructose diet, ESE – elicited soybean extract, Sim – simvastatin 2.8 mg/kg BW
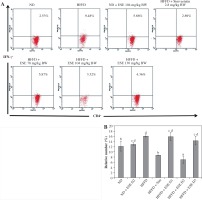
ESE also decrease the expression of IFN-γ in T CD4 cells. The average number of the IFN-γ expression in the mice with a normal diet is 12.22% of the total T CD4 population (Fig. 4A). HFFD mice had significantly increased IFN-γ expression compared to the mice with a normal diet; up to 16.25% of the total T CD4 population. Statistical analysis suggested that (Fig. 4B) the reduced expression of IFN-γ in the HFFD mouse model was significant at all doses (p < 0.05). Expression of IFN-γ in the HFFD mouse model administered with simvastatin was significantly reduced compared to the HFFD mouse model.
Fig. 4
The Expression of IFN-γ after elicited soybean extract (ESE) administration. A) Flow cytometry analysis showed the expression of IFN-γ on CD4 T cells was decreased after administration with the normal dose of ESE. B) The graph is a calculation of a relative number of CD4+ T cells expressing IFN-γ on spleen cells after ESE treatment for four weeks. Data are mean ±SD values of each group p ≤ 0.05. The difference of abjad indicated a significant difference by Duncan test. D1 – ESE 78 mg/kg BW (low dose), D2 – ESE 104 mg/kg BW (normal dose), D3 – ESE 130 mg/kg BW (high dose), ND – normal diet, HFFD – high-fat and -fructose diet, ESE – elicited soybean extract, Sim – simvastatin 2.8 mg/kg BW
Discussion
Obesity is caused by an increasing amount of lipid components, primarily triglycerides, in the adipose tissues, causing adipocyte to hypertrophy and hyperplasia [4]. Hypertrophy of adipocyte leads to the death of the cell [5]. The dying adipocytes trigger an increase in adipokines including TNF-α, IL-6, leptin, visfatin, resistin, angiotensin II, and plasminogen activator inhibitor 1 in the fat tissues [6].
The conditions of obesity can activate TLR4 through endogenous dangerous signals (DAMP) from dying adipocytes and through the increase of fatty acid levels [10, 24]. TLR3 can also be activated once it converges with ligands, such as DAMP, which are produced by dying adipocytes under obesity conditions and eventually increase the severity of the obese condition [10, 25]. Obese people have TNF-α mRNA expression two to three times higher than normal, but this will decrease with weight loss [26, 27]. IFN-γ will increase also under obesity conditions because the activated CD4 T cells in the fat tissue produce more IFN-γ than normal [28]. The increase in pro-inflammatory cytokines will worsen the inflammation, so it should be suppressed.
This study uses elicited soybean extracts (ESE) to suppress the inflammation that occurs in the HFFD mouse model because the content of secondary metabolites (isoflavones such as genistein, glyceollin, and daidzein) is higher than in un-elicited soybean extract [13]. Isoflavones of soybean play a role as an immunoregulator and anti-inflammatory agent [29, 30].
The results showed a significant increase in the relative number of T CD4+TLR3+ and CD4+TLR4+ cells in the HFFD mouse model compared to the normal diet group (Fig. 1B and Fig. 2B). The expression of TLR3+ and TLR4+ in T CD4+ cells in the HFFD mouse model were decreased after ESE administration for four weeks. Isoflavones in soy extracts may inhibit the activation of the TLR. Genistein is able to suppress inflammation induced by LPS in BV2 microglial cells by blocking the binding site between LPS and TLR4, thus inhibiting activation of NF-KB in the production of pro-inflammatory cytokines [31]. Allegedly, the genistein of ESE in this study can disrupt the bond between TLR4/TLR3 with free fatty acids or DAMP produced by dying adipocytes in the HFFD mouse model.
The results showed that a normal dose of ESE significantly decreases the production of TNF-α and IFN-γ by CD4+ T cells in the HFFD mouse model compared to the normal-diet mice (Fig. 3B and Fig. 4B). Genistein contained in the extract acts as an inhibitor of protein tyrosine kinase, which in in vitro studies has been shown to inhibit the NF-kB’s TNF-α producing activity in mice with a high-fat diet [32]. In addition to being able to decrease the expression of TNF-α, genistein is also able to reduce the production of IFN-γ in mice encephalomyelitis after oral administration [33]. The structure of soybean’s genistein is similar to 17β-oestradiol, enabling it to act like oestrogen 2 or as an antagonist to oestrogen 2, which can suppress inflammation [34].
Daidzein has been confirmed to decrease the amount of cholesterol, LDL cholesterol, and free fatty acids in the plasma and liver of mice with a high-fat diet [35]. Meanwhile, glyceollin has been found to inhibit the activity of NF-kB, which plays a role in inflammation, so that the production of pro-inflammatory cytokines, such as TNF-α and IFN-γ, were also reduced [36]. Propolis is also able to suppress the production of pro-inflammatory cytokines through increased regulatory T-cell activation [37].
Administration of simvastatin with a dose of 2.8 mg/kg BW can decrease the expression of TLR3+ and TLR4+ in T CD4+ cells of the HFFD mouse model. According to [38], simvastatin is able to suppress inflammation in the adipose tissues of obese mice by inhibiting the expression of TLR3 and TLR4. Inhibition of the expression of TLR4 can suppress the activation of interferon regulatory factor (IRF) 3, leading to inhibition of IFN-β expression. Neutralisation of IFN-β expression can decrease the activity of pro-inflammatory cytokines. Simvastatin reduced TNF-α expression, which was consistent with the previous study, which demonstrated that simvastatin can lower the mRNA expression that encodes TNF-α in fat tissues [39]. Simvastatin also acts as an inhibitor of IFN-γ activation [40]. These results lead to a reduction of the risk of inflammation.
Conclusions
The elicited soybean extracts (ESE) with a normal dose (104 mg/kg BW) can decrease the expression of inflammatory mediators such as TLR3 and TLR4 as well as pro-inflammatory cytokines TNF-α and IFN-γ. Therefore, ESE in a normal dose can be used as an alternative medicine in the treatment of obesity and as an anti-inflammatory agent.


