Fluoroquinolone (FQ) antibiotics are often used in medical therapy, especially in the treatment of urinary tract infections (UTIs). Recently, serious adverse effects associated with their use have increasingly been reported. The US Food and Drug Administration (FDA) Adverse Event Reporting System (AERS) database includes 210,705 adverse events (and 2,991 fatalities) related to the use of FQs, as of January 2016 [1]. The most common adverse drug reactions (ADRs) associated with the FQs involve the gastrointestinal tract (nausea and diarrhoea), the central and peripheral nervous system (headache, dizziness, a “pins and needles” tingling or pricking sensation, confusion, hallucinations, and suicidal thoughts). Other potentially serious events involve the cardiovascular system (QT interval prolongation), musculoskeletal system (arthropathy, tendinopathy, tendon rupture, muscle weakness, joint pain and swelling) and glucose homeostasis dysregulation [2].
The FDA first added a black-box warning label for FQs in July 2008 regarding the increased risk of tendinitis and tendon rupture. In August 2013, the agency required new updates to the labels to describe the potential for irreversible peripheral neuropathy. In 2016 the FDA strengthened these warnings and emphasised the association of FQs with disabling and potentially permanent serious side effects, the risks of which generally outweigh the benefits in treatments for acute bacterial sinusitis, chronic bronchitis and uncomplicated urinary tract infections. In their most recent update of July 2018, the FDA required the drug labels to state that FQs may cause significant decreases in blood sugar and certain mental health side effects.
FQs are widely used in clinical practice despite the FDA’s recommendations restricting the use of these antibiotics. Unfortunately, this means the number of patients suffering from serious and chronic side effects of FQs is still growing. In addition, there is a lack of causative treatment at the current time. Disease-modifying and symptomatic treatment is complex, difficult and not standardized.
We present the case of 43-year-old man, previously in good health, who experienced severe ADRs 5 days after completion of levofloxacin treatment, and was finally diagnosed with fluoroquinolone-associated disability (FQAD). In 2015, for urological reasons, the patient underwent empirical, prolonged antibiotic therapy including a 24-day course of levofloxacin : 1500 mg (p.o.) daily for the first 3 days and 500 mg daily for next 21 days. Five days after treatment completion, the patient started to suffer from the first side effects. There were a number of different areas affected, including the central and peripheral nervous system (paraesthesia, speech and vision disorders, “pins and needles” sensation, chronic fatigue, anxiety, confusion, brain fog, memory loss and depression) and the musculoskeletal system (severe upper and lower limb pain, unusual neck pain, muscle weakness). Serious tendon, muscle and joint injuries were subsequently diagnosed. Additionally, gastrointestinal tract (uncommon food sensitivities), urinary tract and skin complaints were reported. During the next 3 years the patient underwent many specialist consultations in Poland, as well as in China and the USA.
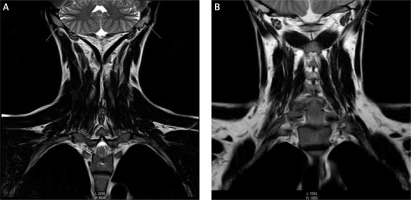
A number of laboratory, functional and imaging tests were carried out. An electromyographic examination (EMG) in April 2015 revealed peripheral nerve demyelination. Magnetic resonance imaging (MRI) in September 2016 revealed bilateral degeneration of the forearm proximal tendons and degenerative changes of the neck muscles, with progression of these changes shown in the study of March 2018 (Figure 1). An earlier MRI study of April 2016 did not reveal any changes despite persistent clinical symptoms. Ultrasonography (USG) in September 2016 showed FQ-associated bilateral Achilles and forearm flexor tendinopathy. Bilateral partial rupture of the Achilles tendon was confirmed by MRI. An earlier USG examination of April 2016 did not reveal the described injuries despite the occurrence of constant pain. A number of other tests were also carried out, including the Organic Acids Test, which assess the intestinal bacterial flora and the level of mitochondrial markers of the Krebs cycle. Biochemical tests revealed a decreased level of neurotransmitters: dopamine, norepinephrine, epinephrine, glutamate, GABA and serotonin. Increased concentrations of vitamin B12 in serum (1289 pg/ml) (norm: 197–771 pg/ml), folic acid (30.9 ng/ml) (norm: 3.1–17.5) and homocysteine (14.73 μmol/l) (norm: 5.00–13.90) were detected. Genetic tests performed in 2015 confirmed MTHFR A1298C, (MTHFR C677T polymorphism wasn’t detected) MAO A/R297R, and CYP3A4 gene polymorphisms. Other routine blood and urine tests were generally non-contributory.
Figure 1
Cervical spine MRI: FSE T2 (A), T1W (B) sequences. Degenerative changes present at proximal attachment sites of the splenius muscles and longissimus capitis
Overall, following the levofloxacin treatment, the patient was repeatedly hospitalised in neurological and orthopaedic wards over a period of 3 years. Additionally, he was referred to specialists including a cardiologist, ophthalmologist, rheumatologist, neurosurgeon, urologist, acupuncture (pain management) specialist and physical therapist. The various multiple injuries and damage made him unable to work.
The patient was treated with medication aiming to reduce oxidative damage. Reduction of oxidative stress was achieved by increasing levels of endogenous and exogenous antioxidants and by stabilising mitochondrial energy production. He received intravenous antioxidant therapy: reduced L-glutathione (GSH), L-ascorbic acid, α-lipoic acid, ubiquinone (coenzyme Q10). The antioxidant therapy was combined with other compounds (e.g. magnesium sulphate) – essential in cellular metabolic processes as well as in the synthesis of adenosine triphosphate (ATP). Achilles tendinopathy was treated with mesenchymal stem cells and repeated platelet-rich plasma injections. Hyperbaric oxygen therapy (HBOT) improved tendon healing, neurological symptoms, physical and mental health. Although after 3 years the patient’s quality of life is better, many environmental factors can induce intermittent episodes of symptoms. In particular, the patient is suffering from multilevel degenerative disc disease, Achilles and other tendinopathies, peripheral and small fibre neuropathy, and also chronic fatigue and headaches.
The cause of the FQAD is multifactorial and is the result of complex interactions including mitochondrial DNA (mtDNA) mutation, respiratory chain dysfunction and increased oxidative stress from mitochondrial damage [3–6]. In the recent published article mtDNA was identified as a key target for ciprofloxacin-induced adverse effects through inhibition of both type II topoisomerases (Top2α and Top2β). The loss of topoisomerase II or its inhibition by fluoroquinolones results in accumulation of positively supercoiled mtDNA, followed by cessation of mitochondrial transcription and replication initiation, causing depletion of mtDNA copy number. These mitochondrial effects block both cell proliferation and differentiation [7]. Moreover FQs can possibly survive inside the cell for many years, resulting in chronic long-term adverse reactions in patients. Michalak et al. indicated some potential methods of reversing FQ side effects, such as: reduction of oxidative stress, supplementation of uni- and bivalent cations that are chelated by FQs, and removing FQs that have been permanently accumulated in the cells [3]. However, it should be emphasised that a standardised way of dealing with the FQ toxicity and FQAD is currently not known.
The main substance that can help, by reducing oxidative damage regardless of the pathophysiology of damage, is naturally occurring glutathione. This antioxidant is well known to minimize the lipid peroxidation of cellular membranes and other such targets that are known to occur with oxidative stress [8]. However, more research and evidence are needed to support its usage for FQAD.
Uivarosi and Seedher et al. have suggested the use of magnesium, which is a natural competitor of FQs for protein binding sites [9, 10]. Supplementation of magnesium has been proven to decrease the absorption of FQs in the gut and can also significantly reduce inflammation [11, 12]. In addition, it seems that ozone can be used to reduce the accumulation of FQs [13].
We still do not know which group of patients is predisposed to adverse reactions after taking FQs. The genetic predisposition for increased risk of FQAD is not yet understood and further research is needed. A possible link between MTHR gene C677T and A1298C polymorphism associated with hyperhomocysteinaemia should be elucidated.
In conclusion, the overuse of FQs and the growing number of reports of chronic adverse reactions (ADRs), including potential FQAD, are the main reasons to avoid FQs when other safer alternatives are available. Rigorous genotoxicity testing and further research must be continued and a “FQ risk assessment” should be a regulatory decision factor for whether to prescribe or not. Additional studies are necessary to determine which genetic factors underlie susceptibility and the genotypes predisposing to FQAD. There is an immediate need to perform preclinical studies and clinical research for the potential benefits and risks of high-dose antioxidant therapy, application of stem cells and other treatment strategies for FQAD.



