Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
ONCOLOGY / LETTER TO THE EDITOR
Arachidonic and eicosapentaenoic acids induce oxidative stress to suppress proliferation of human glioma cells
1
BioScience Research Centre, GVP College of Engineering Campus, Visakhapatnam, India
2
NIPER, Hyderabad, India
3
UND Life Sciences, USA
Submission date: 2018-06-21
Final revision date: 2018-07-20
Acceptance date: 2018-07-20
Online publication date: 2020-01-15
Publication date: 2020-05-26
Arch Med Sci 2020;16(4):974-983
KEYWORDS
TOPICS
ABSTRACT
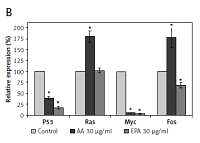
Polyunsaturated fatty acids (PUFAs) suppress and induce apoptosis of tumor cells by enhancing free radical generation and accumulation of excess of lipid peroxides. In the present study, we showed that both AA and EPA (AA > EPA) inhibited proliferation of LN229 and HNGC2 (LN229 > HNGC2) cells and suppressed p53, Ras, Myc, BCL-2/Bax, NF-kB/IkB and 5-LOX and enhanced cytochrome C and caspases 3 and 9 expressions and increased the accumulation of lipid peroxides and nitric oxide and altered their antioxidant defenses (LN229 > HNGC2). These studies suggest that accumulation of lipid peroxides seems to be the principle mechanism by which AA and EPA bring about their growth inhibitory action on tumor cells.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.