Introduction
According to epidemiologic research, trauma of the upper limb in tennis players represents 30–39% of all injuries [1, 2]. Shoulder injury is the most frequent one and it is typically accompanied by pathology of the supraspinatus and the infraspinatus as well as the impingement syndrome [2]. Between 7% and 30% of all injuries to tennis players are shoulder problems [3, 4].
The prevalence of shoulder pain rises with age, especially in highly developed societies where people perform more and more physical activities as a pastime. Those disciplines which include throwing and overhead activity, such as volleyball, handball and tennis, are the ones which cause injuries most often [3, 4].
Frequently performed tennis strokes starting at a young age impact formation of bones and lead to a change of tension and rebuilding of the soft tissues. It results in an increase of the range of external rotation (ER) and a decrease of internal rotation (IR) of the glenohumeral joint on the dominant side [5–8].
In 2013 Kibler et al. [9] defined a limitation of the range of IR of the glenohumeral joint (glenohumeral internal rotation deficit – GIRD) as asymmetry within IR between the sides which is more than 18°, and an insufficiency of ER (external rotation deficiency – ERD) as a lack of increase of the range of ER on the dominant side of at least 5° in comparison to the non-dominant shoulder.
Wilk et al. [10, 11] suggested comparing the sum of IR and ER of the glenohumeral joint (total rotational motion – TROM) of both sides. TROM deficit was defined as the difference between the sides greater than 5°.
There is research evidence showing that specific changes of the range of rotation of the glenohumeral joint are more often observed in overhead athletes feeling shoulder pain [7, 12, 13]. As described in medical literature, the pathologic cascade of the thrower’s shoulder starts with acquired asymptomatic posteroinferior capsular contracture (which restricts internal rotation), continues through the phase of painful shoulder (which is often neglected) and could lead to serious structural changes of the rotator cuff and the articular labrum [5]. To the authors’ knowledge there is a lack of research analyzing exactly in which structures of the shoulder there are pathological changes in the situation when a tennis player complains about shoulder pain or when one has alterations in the glenohumeral range of motion.
Aim
The aim of this study was to evaluate whether using ultrasound examination we can identify tennis players with shoulder pain and those having specific changes of the range of rotation of the glenohumeral joint.
Material and methods
Subjects
Sixty-six tennis players were examined: 42 (63.6%) men and 24 (36.4%) women.
Cases of shoulder surgery, adhesive capsulitis, dislocation and fracture of the scapula or humeral bone were excluded from the study.
The study received the positive opinion of the Bioethics Committee of the Medical University of Warsaw.
Each of the patients was informed in writing about the study procedure. A patient or, in the case of minors, parents signed consent to undergo the study and filled in a form including exclusion criteria.
Medical history
The standardized Visual Analog Scale (VAS) was used to assess shoulder pain during and after playing tennis. The duration of shoulder pain was also determined.
Range of rotation of the glenohumeral joint
Examination of the range of IR and ER of the glenohumeral joint was performed. Right and left limbs were tested at random. Measurement of IR and ER was performed in a sequence tailored to each subject. The examiner was unaware of the examinee’s handedness and the group to which he or she belonged. The measurement was taken at 90o of abduction with a digital inclinometer (PRO 360 Digital Protractor), following the procedures described in previous literature [14–17].
Jobe’s test
To identify shoulder pain and weakness we used Jobe’s test. The examiner positioned the subject’s glenohumeral joints in internal rotation and abduction in scaption (the scapular plane), following the suggestions of the test authors [18, 19]. Next, the examiner pressed the subject’s wrists towards the ground with his hands. The subject was supposed to hold the taken position. The test was considered positive when there was pain and/or weakness.
Neer’s test
To identify shoulder pain we also used Neer’s test. The examiner used one hand to stabilize the subject’s scapula, and the other to perform a passive elevation through flexion and sustaining the glenohumeral joint in internal rotation – according to Neer’s description from 1983 [20]. Next, elevation was performed in scaption. The test was considered positive when there was pain.
Ultrasonography
To assess the rotator cuff, the subacromial bursa and to define the prevalence of subacromial impingement we performed ultrasonography (US) of the dominant shoulder. The radiologist was unaware of the patient’s shoulder condition (painful or not painful).
Toshiba Aplio MX apparatus was used in all examinations. The apparatus was equipped with a linear transducer of 5–18 MHz. The examination was performed by three highly qualified ultrasonographers (doctors). The protocol of the examination was the result of the cooperation of all three specialists (Table I).
Table I
Shoulder ultrasound examination protocol
The examination analyzed the state of the supraspinatus (SSP), infraspinatus (ISP) and subscapularis (SSC) tendons with capsuloligamentous complex. The teres minor was not taken into consideration because its damage is extremely rare in tennis players.
During the examination the specialists assessed pathologies of tendons of the examined muscles (full or partial rupture), capsuloligamentous structures (full or partial rupture), entheses of tendons (scars, erosions or calcific cavities) and entheses of capsuloligamentous structures (scars, erosions or calcific cavities).
In the subacromial bursa the presence of an increased amount of liquid (effusion), synovial swelling and fibrosis was examined as a feature of acute, chronic or post-inflammatory condition.
We used the phrase “pathological shoulder changes” for any deviation from the norm for the SSP, ISP, SSC and the subacromial bursa.
The presence of signs and symptoms of subacromial impingement (such as the greater tubercle of the humerus not being pulled in fully under the acromion or presence of pain) were examined during passive and active abduction of the glenohumeral joint within 0–90o.
Statistical analysis
The calculations were done using the STATISTICA 12.0 PL program (StatSoft, Inc. 2015, Tulsa, USA). Statistically significant results were those where making type I error probability was less than 5% (p < 0.05). Data analysis used both parametric and non-parametric tests (depending on analyzed data distribution).
The auxiliary calculations, tables and graphs were prepared with MS Excel (Microsoft Corporation 2010, Redmond, USA).
In reference to detecting the pathology of given shoulder structures we also calculated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy together with 95% confidence intervals for Neer’s test and Jobe’s test.
Results
The subjects were divided into two groups (the study group and the control group).
The study group consisted of 37 players: 22 (59.5%) men and 15 (40.5%) women suffering from shoulder pain, aged 11–40 (mean: 24.2 ±8.6) and who play tennis at least twice a week, for 2 previous years minimum. The group included tennis players experiencing shoulder pain in at least one of the situations during the period of the previous 12 months – while playing tennis and directly after/the day after playing tennis.
The control group consisted of 29 tennis players: 20 (69.0%) men and 9 (31.0%) women without shoulder pain, aged 10–39 (21.9 ±10.8), playing tennis at least twice a week for the previous 2 years or more.
There were no significant differences in age and sex in both groups.
Shoulder pain
In the study group shoulder pain felt during playing tennis was estimated at 3.8 ±2.2 in the VAS (0–10). Fifty-four percent of subjects claimed that the condition was equal or more than 4 in the VAS. Shoulder pain which occurred after playing tennis was estimated at 3.4 ±2.4. 43.2% of tennis players suffered from this kind of pain at the level of 4 or more in the VAS.
Subjects with significant pain were defined as those reporting the level of shoulder pain in the VAS within the range 4–10, both during and after play. Subjects without significant pain were those suffering from shoulder pain in both situations within the range 0–3. In this way we missed some tennis players complaining about pain within the range 4–10 only in one of the mentioned situations, but probably it increased the chance to reveal possible relations.
It was not confirmed that the duration of shoulder pain (< 1 month, 1–3 months or > 3 months) was significantly correlated either with the occurrence of pathological shoulder changes or with the occurrence of features of subacromial impingement (Table II).
Table II
Relationship between pathological changes of the shoulder and features of subacromial impingement occurrence vs. duration of shoulder pain; Spearman’s rho correlation coefficient
| Variable | Duration of shoulder pain | |
|---|---|---|
| rho | P-value | |
| Incidence rate | 0.203 | 0.102 |
| SSP | 0.181 | 0.145 |
| ISP | 0.197 | 0.113 |
| SSC | 0.151 | 0.227 |
| Bursa | –0.062 | 0.621 |
| Impingement | 0.174 | 0.163 |
On the dominant side Jobe’s test was positive among 43.2% of the study group and 0% in the control group. On the non-dominant side the test was negative in all subjects. On the dominant side Neer’s test was positive among 54% of the study group and 0% in the control group. On the non-dominant side the test was negative in all subjects (Table III).
Table III
Relations between values, prevalence and incidence of studied features vs. group
Range of motion
Comparing the study and the control groups we identified a statistically significant difference (p = 0.030) in the range of IR of the glenohumeral joint on the dominant side: 47.5 ±11.3° and 53.6 ±10.9° respectively. There was a non-significantly (p = 0.084) smaller TROM on the dominant side in the study group (146.0 ±17.3°) in comparison to the control group (152.8 ±13.3°) (Table III).
After including the side of measurement the analysis of variance in the intragroup scheme showed a significant difference between IR and ER (p < 0.001) – IR was bigger on the non-dominant side, while ER was bigger on the dominant side (Figure 1).
Figure 1
Range of external and internal rotation (± SE) depending on the side (dominant or non-dominant)
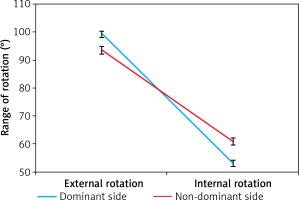
The GIRD, TROM deficit and ERD prevalence did not relate to the group (p > 0.05). The GIRD, TROM deficit and ERD were identified in 17%, 58% and 55% of tennis players respectively (Table IV).
Table IV
Relations between GIRD, TROM deficit and ERD prevalence vs. group
Ultrasonography findings
It was observed that 72.7% of subjects had pathological shoulder changes. The maximum number of pathological shoulder changes was 4 (mean: 1.73; SD = 0.89; median = 1). Any pathologies visible in ultrasonography of the SSP, ISP or SSC along with their tendons, entheses and capsuloligamentous structures were more often identified in the study group (59.5% of the subjects) than in the control group (34.5%). It was a statistically significant difference (p = 0.044). There was a non-significantly higher incidence of pathologies of the ISP in the study group than in the control group (p = 0.072). Pathologies of the entheses of capsuloligamentous structures were present in 28 subjects while pathologies of the entheses of tendons were present in 9 subjects. There were 2 partial ruptures of the tendons (SSP and SSC) and also 2 partial ruptures of the capsuloligamentous structures (ISP and SSC) at any place apart from the entheses. Further examination of the entheses pathologies revealed 38 scars and 25 erosions. A calcific cavity was present in 1 person. Within the entheses of capsuloligamentous structures 52 and within the entheses of tendons 11 changes were diagnosed. None of the subjects was identified to have a full rupture of the tendon or capsuloligamentous structure. Pathologies within the bursa occurred in 37.8% of the study group members and 44.8% of the control group. We diagnosed 21 cases of fibrosis and 13 cases of increased amount of liquid or swelling. None of the subjects had subacromial impingement understood as the lack of full abduction (the greater tubercle of the humerus not being pulled in fully under the acromion). Passive and active abduction of the glenohumeral joint performed during the examination of the subacromial impingement under ultrasonography guidance was painful in 3 cases of the study group and in none in the control group (p > 0.05) (Table III).
The incidence rate of pathological shoulder changes was positively correlated with ageing but was not correlated with Neer’s and Jobe’s test results, the ranges of rotations of the glenohumeral joint, the time span of the pain or the intensity of the pain (Table V).
Table V
Correlation between Neer’s and Jobe’s test results, ranges of rotations, age, time span of the pain and intensity of the pain vs. incidence rate of pathological shoulder changes (number of pathological shoulder changes per subject); Pearson’s correlation coefficient (r)
| Variable | Incidence rate |
|---|---|
| Neer D | 0.098 |
| Jobe D | 0.0109 |
| Internal rotation D | –0.163 |
| External rotation D | –0.054 |
| Sum of rotations D | –0.157 |
| Age | 0.385* |
| Time span of pain | 0.149 |
| Pain during play | 0.117 |
| Pain after play | 0.084 |
The relation between the pathologies and the range of rotation of the glenohumeral joint
In the context of the range of rotation of the glenohumeral joint and the prevalence of pathologies of the SSP, ISP or SSC we did not find significant differences (p > 0.05) between the groups of tennis players with significant shoulder pain (VAS 4–10) and those without significant shoulder pain (VAS 0–3) (Table VI).
Table VI
Relation between range of rotation of the glenohumeral joint on dominant and non-dominant sides and prevalence of pathology of SSP, ISP or SSC vs. occurrence of significant pain among tennis players with shoulder pain
The occurrence of GIRD and TROM deficit was independent of pathological shoulder changes. The occurrence of ERD was independent of most pathological shoulder changes. We only observed a significantly higher percentage of people with SSP pathologies in the ERD group (Table VII).
Table VII
Relation between pathological shoulder changes prevalence vs. GIRD, TROM deficit and ERD occurrence
The Mann-Whitney U test analysis did not confirm the dependence between the occurrence of pathologies of the SSP, ISP or SSC and range of internal and external rotation on the dominant side. The pathologies of the subacromial bursa did not influence the differences within external and internal rotation on the dominant side (Table VIII).
Table VIII
Relation between range of internal and external rotation vs. occurrence of pathologies of SSP, ISP, SSC and subacromial bursa
Diagnostic value of Jobe’s test and Neer’s test – the assessment
The following parameters were taken into consideration when assessing the diagnostic value of both tests: sensitivity, specificity, positive predictive value, negative predictive value and test accuracy. The dominant side was analyzed. Ultrasonography was used to identify any shoulder pathologies. Both tests are assessed below.
In Jobe’s test, sensitivity (describing the test ability to detect pathology) had the highest score for the pathology of tendon entheses (44.4%) and the lowest for the pathology of capsuloligamentous structure entheses (25.0%). Specificity did not reveal differences between given structures; a slightly higher result was found for the pathology of SSP, ISP or SSC. The lowest positive predictive value was found for the pathology of tendon entheses (25%). For the remaining pathologies the positive prognostic value was similar.
The assessment of the diagnostic value was also supported by the evaluated accuracy parameter, which indicates the percentage of the correct test classification for the given structures. The accuracy index was crucial for the assessment of the diagnostic value. The highest accuracy was found for tendon entheses pathology (74.2%) – such a high result may be explained with the high negative prognostic value (90%). The remaining structures showed similar test accuracy. Table IX summarizes the results of Jobe’s test.
Table IX
Assessment of diagnostic value of Jobe’s test
For Neer’s test as far as sensitivity is concerned the highest score was found for the pathology of the SSP, ISP or SSC (34.4%) and the lowest for the pathology of tendon entheses (22.2%). We note that the differences here were not as apparent as in the case of Jobe’s test. Considering specificity, the differences between the given structures were not significant with scores over 66%. The pathology of tendon entheses had the lowest positive prognostic value (10%) and the highest value was found for the pathology of the SSP, ISP or SSC (55%).
The highest accuracy was for the pathology of the entheses of the tendon (62.1%) – the result may be explained with the high percentage of the negative prognostic value (84.8%). For the remaining structures the accuracy of the test was similar. Table X summarizes the results of Neer’s test.
Table X.
Assessment of diagnostic value of Neer’s test
Discussion
This study was performed in a group of tennis players to determine whether US can identify painful shoulders and specific alterations in the range of motion.
An important issue in the context of accuracy is the interpretation of the ultrasonography examination result by a radiologist. The study of Scheel et al. [21] assessed the interobserver reliability among independent radiologists. The modified κ factor of agreement (proposed by Craig [22]) for the shoulder examination was estimated to be 0.76, which was considered a good result. It was concluded that the different interpretation of the image was due to a lack of standards both in the scanning techniques as well as in the description of the results (definitions of pathological findings). In our current study both factors were reduced because the diagnosticians were in fact not independent: they consulted and agreed upon the same protocol, and, in general, shared the same diagnostic practices. We believe that, altogether, such correlation between the examiners positively influenced the consistency of the results.
Considering the facts that none of the subjects was identified with a full rupture of the tendon or capsuloligamentous structure and the incidence rate of the pathological shoulder changes rose with age, it is suggested that the pathologies in this area of the body develop slowly in tennis players.
Rotator cuff is a multilayer structure built of interweaving fibers of tendons and capsuloligamentous complex. The outer tendon part plays the dynamic role while the inner capsuloligamentous layer controls the joint statically. The thickness of both is similar. The pathological changes of both layers may have a negative influence on the rotator cuff and the glenohumeral joint function. Thus, during ultrasound examination they should be identified and diagnosed separately [23–25].
Most damage of the rotator cuff was on the joint side (capsuloligamentous structures), which is in line with previous results from the literature [26–29]. More precisely, the pathological changes occurred most often in the region of the entheses of the capsuloligamentous structures. It casts doubt on Neer’s hypothesis stating that 95% of all the pathologies of the rotator cuff are due to the irritation by the anteroinferior aspect of the acromion [30]. If this statement is correct, damage of the rotator cuff should occur mainly on the bursa side, so the tendons would be the structures most exposed to injury. A more probable genesis of the pathologies of the structures on the joint side in tennis players is the overload, meaning the inner factors (compressive and shear forces) which cause the degenerative changes (vascularisation disorders, fiber disorientation, fat infiltration, increase in the number of cells) [26, 31, 32]. Ultrasonic findings of a hypoechoic area correlate with the histologic findings described above [33, 34]. It is also worthwhile noting that in such cases as rotator cuff tendinosis, tears may develop due to tendon weakness [35, 36].
A lot of specialists claim that joint side rotator cuff damage is due to a conflict between the SSP and ISP versus the posterosuperior glenoid rim, known as the posterior or internal impingement syndrome [29, 37–39]. It was proved that tightness of the posteroinferior capsule of the glenohumeral joint increased the glenohumeral contact pressure at maximum ER, causing forceful internal impingement [40].
The results of the ultrasound examination suggest that subacromial impingement does not occur in tennis players as often as most specialists think or it happens only during fast, rapid movements of the upper limb which occur on the tennis court but are impossible to simulate in the physician’s office. It is likely that subacromial impingement can be observed in ultrasonography only at an advanced stage.
The pathologies in the subacromial bursa occurred almost as often as in the rotator cuff and with the same prevalence in both groups. The most common finding in the bursa was fibrosis, which could indicate a post-inflammatory condition. This in turn could be the cause of the pain complaints of the shoulder during the last 12 months.
It was suggested by other authors that the stiffness, shortening and scar formation of the posteroinferior area of the glenohumeral joint (joint capsule, ligaments, muscles and fascia) caused the migration of the humeral head, which in turn might lessen the subacromial space and increase the peel-back forces on the labrum while performing the serve [5, 41–44]. Such a phenomenon as limitation of the glenohumeral joint movement, which itself is not a pathology, may cause overload and damage of the shoulder structures.
It was also proved that not every decrease of IR was pathological and did not have to correlate with an increased likelihood of injury of the shoulder. In overhead athletes GIRD on the dominant side is a natural phenomenon to be expected [12, 15]. It is highly probable that such physiological limitation of IR on the dominant side in tennis players is linked to the increase of the angle of retroversion of the humeral head. Probably pathological GIRD is linked to the tightness of soft tissues. The studies and observations of Burkhart et al. [45] suggested that as long as the limitation of IR was lower or equal in the gain of ER the throwing shoulder had unaltered kinematics and it functioned correctly.
The current study confirms that a common feature for the tennis players with and without shoulder pain is that they have significantly less IR and more ER on the dominant side as compared to the non-dominant. It indicates that this change in the range of motion could be tennis-related. The results do not unambiguously link GIRD with shoulder pain, as other authors proposed [7, 13], but still it reveals that the range of IR on the dominant side is significantly smaller in tennis players with shoulder pain. In the study group there was non-significantly smaller TROM on the dominant side as compared to the control group. We conclude that prevalence of GIRD, TROM deficit and ERD were independent of the group and that the ranges of the rotations were independent of the occurrence of significant pain.
Based on the results, the relation between the shoulder structure and the range of motion is not clear. There was no correlation between the incidence rate of pathological shoulder changes and the ranges of rotations of the glenohumeral joint. The range of internal and external rotation on the dominant side was the same in the people with SSP, ISP, SSC or bursa pathologies as in the people without it. The occurrence of GIRD, TROM deficit and ERD was independent of the prevalence of pathological shoulder changes. There was only one exception: a higher percentage of people with SSP pathologies was present among the group with ERD. Therefore, in tennis players special attention should be paid to mobilization of the range of ER on the dominant side.
Jobe’s test is used in the diagnostics of damage of the supraspinatus [31]. The methodology of performing Jobe’s test needs the subject’s muscle force to keep the upper limbs still. As the entheses of tendons may undergo more strain during muscle contraction than non-contractile capsuloligamentous structures, it is not surprising that this study presented the highest sensitivity (44.4%) and accuracy (74.2%) compared to pathologies of other structures.
Neer’s test is the tool universally used to identify subacromial impingement. In our study Neer’s test was predominantly positive on the dominant side (54% of subjects from the study group). Subacromial impingement, understood as the greater tubercle of the humerus not being pulled in fully under the acromion during abduction, was not identified in anyone in the ultrasonography examination (only 3 subjects had features of impingement in the form of pain during abduction). Hence, subacromial impingement was more often identified using orthopedic tests than in ultrasonography examination. A possible explanation is that Neer’s test is more challenging for the soft tissues (applying extrinsic pressing force after reaching the ending position in full elevation) than ultrasonography (active and passive abduction of the glenohumeral joint within 0–90°) when trying to identify subacromial impingement.
The accuracy of Neer’s tests for the pathology of bursa, SSP, ISP or SSC and entheses of tendons or capsuloligamentous structures was similar for all (between 50.0% and 62.1%). This result may be explained by the fact that all of these tissues are compressed by the acromion and/or the glenoid rim in the ending position of the test and could be the source of the reported pain [46].
Clinical reviews report that the currently practiced shoulder clinical tests target either sensitivity or specificity, and those having both parameters at a high level are lacking [47, 48]. In our study, the sensitivity of Neer’s and Jobe’s tests was lower than the specificity, which shows an ability to identify healthy people rather than those with pathologies (both tests were negative in all subjects from the control group). Due to the low accuracy of the clinical tests for rotator cuff and subacromial impingement, these tests are insufficient to state the correct diagnosis. It is advisable to use ultrasonography for a more precise diagnosis.
Lewis [49] observed no correlation between the patients’ complaints and the structural pathology identified with the imaging tests. The results of the current study confirm this observation to some extent. The prevalence of pathologies of the rotator cuff was higher in the study group than in the control group. On the other hand, the p-value for this relation (0.044) was close to the cut-off value for significance and the difference was significant only for the combined pathologies. The prevalence and also the incidence of SSP pathology, ISP pathology and SSC pathology (when analyzed separately) were not significantly different in tennis players with and without shoulder pain. No significant differences were observed in the prevalence of pathological shoulder changes, bursa pathologies and ultrasonographic features of subacromial impingement in tennis players with shoulder pain and those without pain. Another surprising result is the lack of correlation between the incidence rate of pathological shoulder changes versus the intensity of pain during and after play, the time span of the pain experienced and also the results of Neer’s and Jobe’s tests. Furthermore, no significant differences were observed between the tennis players with significant shoulder pain (VAS ≥ 4) versus those without any significant pain (VAS ≤ 3), in the context of the prevalence of pathologies of the SSP, ISP or SSC.
In tennis players shoulder pain and changes of the ranges of rotation of the glenohumeral joint do not have to be caused by structural pathological changes. There was a considerable number of subjects in the group with shoulder pain without any pathological shoulder changes or features of subacromial impingement observed in ultrasonography. It is possible that among those tennis players the source of the pain was a functional problem, for example myofascial trigger points. This might be the missing link in the diagnostic process of pain complaints and decreased function. Active trigger points could imitate damage of the muscles which are overloaded and have increased stiffness, which in turn may explain the limitation of the range of motion [50–53].
Some limitations of the present study should be considered. Firstly the sample size is small, and thus it is difficult to draw a general conclusion. Secondly it is recommended to examine more structures in ultrasonography (for example the long head of the biceps and the acromioclavicular joint).
Conclusions
We examined ultrasonography as a tool in identifying painful shoulder (taking into consideration the presence, intensity and duration of the pain) and alterations of the range of rotation of the glenohumeral joint among tennis players.
We conclude that ultrasonography could be helpful in detecting tennis players with painful shoulder having SSP, ISP or SSC pathologies. However, it seems that the ability of the method to identify players having specific changes of the range of rotation of the glenohumeral joint is limited, with the exception of tennis players with ERD having SSP pathologies.
In ultrasonography it is possible to identify features of even small inflammation (effusion, synovial swelling) and degenerative changes of the shoulder (scars, erosions or calcific cavities), which might be asymptomatic. Accordingly, US diagnostics could be used to adjust training loads for a tennis player early, that is before serious structural pathological changes develop, such as full rotator cuff tear.
In order to confirm the present results it is necessary to conduct prospective and randomized studies with larger groups over a period of several years.









