Introduction
Hypertension is a major risk factor for a variety of cardiovascular diseases (CVD) including coronary heart disease, ischemic stroke and heart failure [1–5]. Multiple clinical trials and meta-analyses have demonstrated that reducing blood pressure (BP) with medications reduces the risk of cardiovascular events [6–8].
Interestingly and importantly, epidemiological studies have shown that patients with hypertension are more likely to have hyperuricemia and the underlying mechanisms are multifactorial and are not fully understood yet [4, 9, 10]. Furthermore, prior studies have consistently shown that hypertensive patients with hyperuricemia had higher CVD risk than hypertensive patients with normal serum uric acid (UA) level [11–14].
Notably, allopurinol is commonly used to decrease serum UA level and prevent gout [15]. Through inhibiting xanthine oxidase, allopurinol reduces UA production. In addition, prior studies have shown that increased UA is associated with reduced nitric oxide generation and allopurinol may improve endothelial function and increase nitric oxide generation, which in turn reduce BP [15]. Indeed, prior some studies showed that lowering serum UA level with allopurinol was associated with BP reduction [10, 16], However, to our knowledge, no randomized controlled trials have been conducted to evaluate whether treatment of hyperuricemia is beneficial for reducing BP as well as decreasing cardiovascular events in hypertensive patients with hyperuricemia.
Therefore, we conducted a cross-sectional study and the aim of this study was twofold: 1) to compare prevalent composite CVD including coronary heart disease, ischemic stroke and heart failure between patients with and without anti-hyperuricemia treatment; 2) to evaluate the associations of anti-hyperuricemia treatment and prevalent composite CVD. We believe that the results from this study can provide more evidence to support randomized controlled trials to evaluate whether treatment of hyperuricemia can reduce cardiovascular events in hypertensive patients in the future.
Material and methods
Study design and participants’ enrollment
This was a cross-sectional study and was approved by the Clinical Research Ethic Committee of FuWai Hospital Chinese Academic of Medical Science, Shenzhen, Guangdong, China. All participants were enrolled after informed consent was obtained and all participants were enrolled from FuWai Hospital Chinese Academic of Medical Science, Shenzhen, Guangdong, China. The inclusion criteria were as follows: documented primary hypertension, documented hyperuricemia or treatment with allopurinol for at least 3 months before enrollment. The exclusion criteria were as follows: documented secondary hypertension, or had acute gout flare in the last 3 months, treatment with anti-hyperuricemia medications other than allopurinol, had myocardial infarction, ischemic or hemorrhagic stroke or exacerbated congestive heart failure in the last 6 months, or had abnormal thyroid function (including both hypo- and hyperthyroidism) and glomerular filtration rate (GFR) < 60 ml/min/1.73 m2.
Baseline characteristics collection
Demographic characteristics (age and gender), smoking status, body mass index and documented comorbidities were collected at baseline by two independent investigators. Fasting venous blood was drawn for assessment of serum UA, fasting blood glucose (FBG), total cholesterol (TC), C-reactive protein (CRP) and creatinine levels. Specifically, all these measurements were conducted in the Cardiovascular Central Laboratory using biochemical methods with commercial kits. Serum creatinine level were used to calculate GFR using the Modification of Diet in Renal Disease (MDRD) formula [17]. Current medications used were collected. The dose of allopurinol used in the current study was between 100 mg and 300 mg/daily.
Blood pressure measurement and hypertension definition
Regarding the new ACC/AHA hypertension guideline [18], the definition of hypertension in this study was systolic blood pressure (SBP) ≥ 130 mm Hg and/or diastolic blood pressure (DBP) ≥ 80 mm Hg, and pulse pressure (PP) was calculated as SBP minus DBP. No smoking or caffeine-containing beverage consumption, or use of anti-hypertensive medication was allowed before BP measurement. Patients sat quietly for 15 min with their back supported and the appropriate cuff size was used with the bladder encircling at least 80% of the non-dominant arm (Omron HEM- BP742N 5 series, Tokyo, Japan). The patient’s arm was placed on the desk parallel to the heart level. Three readings with a 1-minute interval between measurements were performed and the last two readings were averaged as BP.
Statistical analysis
All patients were separated into two groups as anti-hyperuricemia and control (without anti-hyperuricemia treatment) groups. Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables were expressed as number and proportion; between-group differences were analyzed by Student’s t test or χ2 analysis as appropriate. Serum UA levels were divided into quartile groups and between-group differences in BPs were evaluated. Logistic regression analysis was used to evaluate the association between anti-hyperuricemia treatment and prevalent composite CVD. Statistical analysis was conducted in SPSS 18.0 (SPSS Inc., Chicago, USA).
Results
Comparisons of baseline characteristics
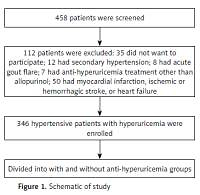
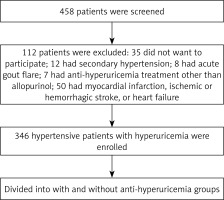
A total of 458 hypertensive patients with documented hyperuricemia or with anti-hyperuricemia treatment were screened and finally 346 patients were enrolled (Figure 1). Baseline characteristics were compared between these two groups. As presented in Table I, compared to the anti-hyperuricemia group, patients in the control group had significantly higher serum C-reactive protein (10.6 ±2.8 vs. 7.4 ±1.2 mg/dl) and UA levels (438 ±33 vs. 379 ±64 µmol/l) but lower GFR (76.6 ±10.2 vs. 80.9 ±11.4 ml/min/1.73 m2). In addition, patients in the control group were more likely to receive β-blocker (34.2% vs. 31.1%) and calcium channel blocker treatment (49.2% vs. 43.4%), resulting in a higher mean number of anti-hypertensive medications used in the control group (2.7 ±0.6 vs. 2.1 ±0.7). The prevalence of ischemic stroke was also higher in the control group (15.8% vs. 11.3%). Furthermore, compared to the anti-hyperuricemia group, SBP (133 ±15 vs. 128 ±13 mm Hg), PP (56 ±12 vs. 50 ±10 mm Hg) and heart rate (80 ±14 vs. 72 ±12 beat per minute) were also significantly higher in the control group. No differences in other comorbidities were observed.
Table I
Comparisons of baseline characteristics
| Variables | Anti-hyperuricemia group (n = 106) | Control group (n = 240) |
|---|---|---|
| Age [years] | 50.8 ±16.7 | 52.4 ±13.5 |
| Female, n (%) | 30 (28.3) | 66 (27.5) |
| Smoker, n (%) | 37 (34.9) | 80 (33.3) |
| Body mass index [kg/m2] | 24.5 ±4.3 | 25.0 ±4.9 |
| Diabetes mellitus, n (%) | 20 (18.9) | 45 (18.8) |
| C-reactive protein [mg/dl] | 7.4 ±1.2 | 10.6 ±2.8* |
| Total cholesterol [mmol/l] | 4.8 ±0.7 | 5.0 ±0.8 |
| Fasting plasma glucose [mmol/l] | 6.0 ±0.5 | 6.2 ±0.8 |
| Uric acid [µmol/l] | 379 ±64 | 438 ±33* |
| Creatinine [µmol/l] | 76.4 ±21.3 | 79.2 ±20.6 |
| Glomerular filtration rate [ml/min/1.73 m2] | 80.9 ±11.4 | 76.6 ±10.2* |
| Statin, n (%) | 29 (27.4) | 70 (29.2) |
| Anti-hypertensive medications, n (%): | ||
| Hydrochlorothiazide | 35 (33.0) | 78 (32.5) |
| ACEI/ARB | 40 (37.7) | 87 (36.3) |
| β-Blocker | 33 (31.1) | 82 (34.2)* |
| Calcium channel blocker | 46 (43.4) | 118 (49.2)* |
| Mean number of anti-hypertensive medications | 2.1 ±0.7 | 2.7 ±0.6* |
| Hypoglycemia medications, n (%) | 16 (15.1) | 42 (17.5) |
| Composite CVD, n (%): | ||
| Coronary heart disease | 7 (6.6) | 17 (7.1) |
| Ischemic stroke | 12 (11.3) | 38 (15.8)* |
| Heart failure | 5 (4.7) | 13 (5.4) |
| Systolic blood pressure [mm Hg] | 128 ±13 | 133 ±15* |
| Diastolic blood pressure [mm Hg] | 74 ±9 | 76 ±11 |
| Pulse pressure [mm Hg] | 50 ±10 | 56 ±12* |
| Heart rate [beats per minute] | 72 ±12 | 80 ±14* |
Comparisons of BPs among different serum UA level groups
Patients were divided into four groups according to the quartile serum UA levels. As presented in Table II, a linear trend was observed between serum UA level and BP levels. Compared to other groups, SBP, DBP, PP and heart rate were all significantly higher in patients of the 4th quartile serum UA level group.
Table II
Blood pressure comparisons between different serum UA levels
| UA levels | SBP [mm Hg] | DBP [mm Hg] | PP [mm Hg] | HR [bpm] |
|---|---|---|---|---|
| 1st quartile (n = 86) | 124 ±14 | 65 ±8 | 45 ±8 | 67 ±9 |
| 2nd quartile (n = 86) | 133 ±13 | 71 ±12 | 49 ±7 | 74 ±11 |
| 3rd quartile (n = 87) | 141 ±16 | 75 ±10 | 55 ±9 | 80 ±10 |
| 4th quartile (n = 87) | 154 ±20* | 80 ±12* | 60 ±12* | 86 ±16* |
Association of anti-hyperuricemia treatment and composite CVD
In order to evaluate the association of anti-hyperuricemia treatment and composite CVD, logistic regression analysis was performed. As presented in Table III, in the unadjusted model, anti-hyperuricemia treatment was significantly associated with reduced odds ratio (OR) of composite CVD. After adjusting for potential covariates, in model 4, the OR of anti-hyperuricemia treatment for composite CVD was 0.89 with a 95% confidence interval (IC) of 0.82–0.98. The associations of anti-hyperuricemia treatment and ischemic stroke were also statistically significant with OR = 0.93 and 95% CI: 0.88–0.99, while the associations of anti-hyperuricemia treatment and coronary heart disease and heart failure attenuated into statistical insignificance after adjusting for covariates.
Table III
Association of anti-hyperuricemia treatment and composite CVD
[i] CVD – cardiovascular disease, CHD – coronary heart disease. Model 1 – adjusted for age, male gender and body mass index, Model 2 – adjusted for model 1 + smoker, diabetes mellitus, total cholesterol, C-reactive protein and GFR, Model 3 – adjusted for model 1 + model 2 + statin and anti-hypertensive medications, Model 4 – adjusted for model 1 + model 2 + model 3 + systolic blood pressure and serum UA level.
Discussion
Hypertension is a major public health burden around the world including China [19, 20]. Many clinical trials have been conducted to evaluate how to better reduce hypertension-related cardiovascular events. Indeed, more aggressive BP reduction (in terms of therapeutic SBP < 120 mm Hg vs. < 140 mm Hg) were associated with lower cardiovascular events as demonstrated by the SPRINT trial [8]. However, observational studies have consistently shown that a proportion of hypertensive patients with increased serum UA level had more cardiovascular events than their hypertensive counterparts with a normal UA level. For example, Alderman et al. [11] reported that among 7978 moderate-severe hypertensive patients, despite BP control, increased serum UA level was significantly and directly associated with cardiovascular events. In another cohort study, Fang et al. [13] reported that baseline serum UA was independently associated with cardiovascular mortality. In a recent prospective study, Kuwabara et al. [21] also demonstrated that increased serum UA level was associated with higher incidence of hypertension among patients with pre-hypertensive status. These findings together strongly indicate that increased serum UA level not only contributed to risk factor development but also was significantly associated with cardiovascular events. Consistent with prior reports, this study also suggested that in the Chinese hypertensive patients, those with higher serum UA level were more likely to have higher BP levels. The underlying mechanisms might be multifactorial. Prior studies have shown that increased serum UA led to endothelial dysfunction, inflammation, oxidative stress and smooth muscle proliferation, together resulting in reduced nitric oxide production, arterial stiffness and BP elevation [22–26].
Unfortunately, to our knowledge, up till now, no clinical trials have demonstrated that lowering serum UA level can improve cardiovascular prognosis of hypertensive patients. This cross-sectional study indicated that in hypertensive patients with increased serum UA level, allopurinol treatment was associated with lower prevalence of composite CVD, and these benefits were mainly driven by lower risk of ischemic stroke. To our knowledge, this was the first study to show that allopurinol treatment was associated with lower risk of ischemic stroke in Chinese hypertensive patients. Notably, the incidence and prevalence of ischemic stroke were significantly higher than those of other atherosclerotic cardiovascular diseases [19]. The underlying mechanism might involve the effects of allopurinol to reduce serum UA level, which in turn led to a reduced inflammatory reaction. Indeed, compared to the control group, serum C-reactive protein level was significantly lower in the anti-hyperuricemia group. These findings suggest that in Chinese hypertensive patients, screening serum UA level and treatment of hyperuricemia may provide great opportunities in reducing CVD burden in China. Further studies are needed to corroborate our findings. In addition, whether findings from this study can extrapolate to other ethnic populations also needs to be evaluated.
Some limitations of this study should be addressed. First of all, the inherent bias of observation study did not allow us to prove a causal relationship between anti-hyperuricemia treatment and risk of prevalent CVD. However, findings from this study provide insight into the association of anti-hyperuricemia treatment and risk of prevalent CVD. Second, this study was conducted in Chinese hypertensive patients and future studies in other ethnic groups are needed to corroborate our findings. Third, although we have adjusted for potential confounding factors, unmeasured and undetected factors still existed which may influence the association of anti-hyperuricemia treatment and risk of prevalent CVD. Last but not least, results from an observational study cannot substitute for a randomized clinical trial, and the clinical relevance of our findings is in providing more evidence to support the notion that reducing serum UA level should be beneficial for prevention of cardiovascular events.
The major findings of this study were as follows: 1) compared to the control group, patients in the anti-hyperuricemia treatment group had lower prevalence of composite CVD, which was mainly driven by the differences in prevalent ischemic stroke; 2) patients with higher serum UA level were more likely to have higher BP levels; 3) anti-hyperuricemia treatment was significantly associated with lower risk of prevalent CVD after adjusting for confounding factors. These findings together indicate that hypertensive patients with hyperuricemia have higher risk of CVD and anti-hyperuricemia treatment may be beneficial for reducing the risk of CVD. Future studies are needed to corroborate our findings.
In conclusion, our preliminary study indicates that in primary hypertensive patients, increased serum UA level is associated with higher BP levels. Furthermore, anti-hyperuricemia treatment is associated with lower prevalence of CVD. Randomized controlled trials are warranted to evaluate whether reducing UA level can improve prognosis of patients with hyperuricemia.