Introduction
Arterial hypertension reflected as increased systolic and/or diastolic blood pressure (SBP and DBP) is a major modifiable risk factor for cardiovascular diseases (CVD) [1, 2] and reduction of BP was associated with reduced cardiovascular events [3, 4]. In addition, it has been well demonstrated that long-term BP elevation is associated with renal function impairment and chronic kidney disease (CKD), which is a common complication of arterial hypertension [5, 6]. Notably, hypertensive patients with CKD have increased risk of CVD versus their hypertensive counterparts without CKD [7]. Therefore, it is clinically important and relevant to identify hypertensive patients who have an early change of renal function so as to prevent CKD development.
Microalbuminuria (MAU) is a sensitive and specific marker to reflect minor and early-stage impaired renal function [8, 9]. It is reported that diabetes mellitus or metabolic syndrome is significantly associated with MAU [10–12]. Nonetheless, the evidence regarding the association between blood pressure components and MAU is limited. In our present cross-sectional study, we enrolled 1858 patients with newly diagnosed hypertension but without previous anti-hypertensive therapy. The aim of our current study was to evaluate whether BP components, namely SBP/DBP and pulse pressure (PP), and MAU after adjustment for potential covariates were independently associated with MAU after adjusting for potential covariates, and also to study which BP component is better associated with MAU. We considered that data from our current study would help us to better understand the association of BP components and early renal function impairment in treatment-naïve hypertensive patients.
Material and methods
Participants’ enrollment
All participants were enrolled from Liaobu Town of Dongguang, Guangdong Province, and informed consent was obtained before enrollment. All the participants were older than 18 years and were newly diagnosed with arterial hypertension in accordance with the diagnostic criteria [13]. Those with secondary hypertension, who had acute coronary syndrome or cerebrovascular diseases in the last 3 months, who were incapable of completing the questionnaire because of intellectual impairment and other conditions, who had other severe diseases with less than 1 year life expectancy, or who were unwilling to take part in the present study, were excluded. A total of 1858 participants with newly diagnosed hypertension and without previous anti-hypertensive therapy were finally enrolled and included in the analysis.
Data collection
Demographics including age and gender, SBP/DBP, PP, body mass index (BMI), waist-hip ratio, and heart rate at rest were extracted from the electronic health record. To be specific, BP measurements were based on the JNC 7 guideline recommendation [14], where patients sat quietly for 10 min and an appropriate cuff size was applied to the non-dominant arm with the bladder encircling at least 80% of the arm (HEM7200, Omron Healthcare, Tokyo, Japan). The patient’s back was supported and the arm was placed on the desk parallel to the level of the heart. Blood pressure measurement was performed 3 times with a 1-minute interval and the last two readings were averaged. Fasting venous blood was drawn for lipid profiles, fasting blood glucose (FBG), serum levels of albumin, creatinine (Cr), blood urine nitrogen (BUN), and uric acid detections.
Urine albumin concentration detection
Patients were instructed on how to collect 24 h urine by trained staff. Specific containers were provided and required storage in a cool environment during urine collection. The first urine in the first morning was discarded and thereafter urine was collected throughout the period until the first urine on the second morning. After that, 24 h urine was sent back to core lab for urine albumin concentration assessment. In accordance with the diagnostic criterion, MAU was defined as 24-hour urine albumin excretion between 30 and 300 mg. On the basis of urine albumin concentration, all participants were divided into the MAU (n = 967) and the normo-albuminuria (n = 891) groups. Comparisons were performed between groups, and the associations between blood pressure components and MAU as well as the sensitivity and specificity of blood pressure components in predicting MAU were evaluated.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median (IQR) appropriately, and between-group differences were tested using Student’s t-test when data were normally distributed; otherwise they were compared using the Wilcoxon rank-sum test. Categorical data were presented as percentage and compared using the χ2 test. Logistic regression analyses and receiver operating characteristic curves (ROC) were performed. Statistical analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). A value of p < 0.05 was considered statistically significant.
Results
Comparisons of baseline characteristics
As presented in Table I, compared to the normo-albuminuria group, patients in the MAU group were older and had higher SBP and PP (p < 0.05 for all comparisons), but DBP was comparable. Additionally, serum levels of FBG, total protein, Cr, BUN and high-density lipoprotein cholesterol (HDL-C) were also significantly higher in the MAU group than the normo-albuminuria group (p < 0.05 for all comparisons). Moreover, 24-hour urine albumin excretion in the MAU group was significantly higher than that in the normo-albuminuria group (182.5 ±156.5 mg vs. 17.6 ±7.1 mg, p < 0.001).
Table I
Comparison of baseline characteristics between groups
Logistic regression analyses
Logistic regression analyses were performed to evaluate the odds ratio (OR) of BP components for MAU in patients with newly diagnosed hypertension but without previous anti-hypertensive treatment. As revealed in the analyses, both SBP and PP were significantly and positively associated with MAU, with OR of 1.010 (95% confidence interval (CI): 1.005–1.016, p < 0.001) in SBP and OR of 1.009 (95% CI: 1.003–1.015, p = 0.003) in PP, respectively. No significant association between DBP and MAU was observed.
Sensitivity and specificity of SBP and PP in predicting MAU
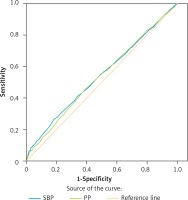
We further used the receiver operating characteristic curve to evaluate the sensitivity and specificity of SBP and PP in predicting MAU. As revealed in Figure 1, the area under the curve for SBP to predict MAU was 0.541 ±0.013 (p = 0.002), and PP was 0.536 ±0.013 (p = 0.006). The difference between SBP and PP in predicting MAU was non-significant, suggesting that the sensitivity and specificity of SBP and PP in predicting MAU were comparable.
Discussion
Renal dysfunction has been recognized as one of the most intractable complications of arterial hypertension, and identifying those who are at increased risk of developing renal dysfunction should confer profound benefits for reducing the incidence of renal dysfunction, resistant hypertension and cardiovascular diseases. Microalbuminuria may be one of the useful and helpful markers in identifying those who are at increased risk of renal dysfunction [15]. Data from our present research indicated that in patients with newly diagnosed hypertension and without previous anti-hypertensive treatment, the prevalence of MAU was 52.1%, suggesting the clinical importance of detection of MAU in these patients as a screening marker for early renal function impairment. Moreover, as analyzed by logistic regression and receiver operating characteristic curve analyses, SBP and PP could be useful for identifying those patients who have MAU and are at increased risk for CKD. However, underlying mechanisms need to be further investigated.
Accumulating evidence has consistently shown that diabetes mellitus and metabolic syndrome are significantly associated with MAU development, suggesting that diabetes mellitus and metabolic syndrome are independent risk factors for CKD development. Managing these comorbidities should be beneficial for reducing and deterring the progression of MAU in these populations. Long-standing arterial hypertension is also known to be an important risk factor for CKD [14, 16]. Nonetheless, the data on the association of newly diagnosed and treatment-naïve hypertension and MAU are limited. Data from our preliminary research showed that in the community population, the prevalence of MAU in the newly diagnosed and treatment-naïve hypertensive patients was up to 52.1%, suggesting that renal injury was one of the commonly encountered complications associated with arterial hypertension and it is critical and essential to routinely perform MAU screening in these newly diagnosed hypertensive patients. However, since it was not possible to identify the exact duration from the beginning of hypertension in this patient population, we could not be certain whether there was a linear relationship between the duration of BP elevation and the degree of MAU.
As regards the value of SBP and PP to predict the prevalence of MAU in the studied patients, logistic regression analyses were performed. After adjustment for traditional risk factors including age, fasting blood glucose, body mass index and lipid profiles, both the SBP and PP were still significantly and independently associated with MAU, suggesting that increased SBP or PP was associated with MAU in treatment-naïve hypertensive patients. To our best knowledge, this finding might be due to the following mechanisms. Generally, increased SBP directly results in increased glomerular pressure and glomerular infiltration rate (GFR). Data from basic research revealed that in rats infused with angiotensin II, intra-glomerular pressure was profoundly increased, which contributed to trans-membrane protein escape and then proteinuria [17, 18]. In addition, increased intra-glomerular pressure could also leads to an increase of membrane permeability, thereby resulting in increased protein trans-membrane excretion [19, 20]. Findings from clinical studies further supported this notion. Lee et al. reported that compared to the normoalbuminuric population, patients with MAU had significantly higher GFR values [21]. Another study also showed that blood pressure levels correlate significantly with MAU in hypertensive patients [22]. However, unlike previous studies, we enrolled newly diagnosed and treatment-naïve hypertensive patients. Therefore, we could exclude confounding effects related to the use of medications such as angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. In addition, our present study revealed, for the first time, the comparable value of SBP and PP in association with MAU in newly diagnosed and treatment-naïve hypertensive patients, suggesting that PP was also an important biomarker reflecting the pathophysiological effects of increased BP on renal function impairment. Moreover, the sensitivity and specificity of SBP and PP in predicting MAU were also comparable, as revealed by the receiver operating characteristic curve.
The clinical implications of our current findings are two-fold: on one hand, it is reasonable and clinically relevant to screen MAU in newly diagnosed and treatment-naïve hypertensive patients; on the other hand, evaluating MAU could be helpful and useful to predict the risk of developing CKD in these populations. Future studies should focus on whether improving BP control, especially SBP and PP, would reduce MAU and thereby deter CKD development.
In conclusion, data from our preliminary research indicate that after adjusting for traditional risk factors, increased SBP and PP are associated with increased prevalence of MAU in newly diagnosed and treatment-naïve hypertensive patients.