Introduction
Adipose tissue is a specialized connective tissue composed of adipocytes. The primary function of adipose tissue is to store energy. Lipids are a good source of energy because they are readily available and easily metabolised, so they can be utilised by the body very quickly [1]. Adipose tissue comprises about 20–25% of total body weight in healthy individuals. In addition to this, it has many other important functions in the human body. These include thermal isolation, cushioning the organs, an endocrine role and production of numerous bioactive factors [2]. The role of adipose tissue has been underestimated in dermatology for a long time. In recent years, a better understanding of adipocyte physiology and its role in human body has opened a new road for aesthetic and interventional dermatology. The adipose tissue from abdominal surgery or liposuction is rich in easily isolated mesenchymal stem/stromal cells (MSCs), which may have a significant role in tissue regeneration. Human adipose tissue-derived mesenchymal stromal cells (hAT-MSCs) are multipotent cells with a high proliferation potential. Many studies proved that MSCs obtained from adipose tissue maintain the ability to differentiate into mesodermal lineages including fat, bone, cartilage and muscle cells. AT-MSCs have also broad pro-angiogenic, anti-inflammatory and anti-apoptotic potential, both in vitro and in vivo. It seems important to understand how one can stimulate an increase in the number of AT-MSCs in the organism with the least interference in the human body [3–6].
Laser radiation, depending on parameters, blocks or stimulates cell processes [7]. The cell response depends on the combination of the wave length, energy, dose and cell type [8]. The lasers used in the therapy, depending on the level of energy, may be divided into those of high power (high intensity lasers – HIL) and those of low power (low-level lasers – LLL). The high intensity lasers (Carbon dioxide, Argon, Erbium:yttrium-aluminium-garnet, Neodymium:yttrium-aluminium-garnet), due to their high level energy, are used in bleeding stoppage, cutting tissues and destroying cells. The low-level lasers (Helium-neon, diode) emit low power and energy used in order to achieve a therapeutic effect. It is connected with a slight increase in temperature (~0.5oC), which does not cause any change to the molecular structure, protein denaturation or apoptosis [9]. Electromagnetic radiation absorbed by chromophores located in cells causes stimulation of biochemical activity of the cell as well as activation of cell metabolism. An increase in production of ATP and in the rate of synthesis of DNA, RNA and proteins takes place [10]. Low-level lasers generate a wavelength of 600–1100 nm, power of 1–500 mW and doses (energy density) of 0.04–50 J/cm2 [11]. Laser treatment is used for healing skin wounds, nerves and cornea regeneration, reconstruction of bone loss as well as for regeneration of cartilage [12–16]. In clinical practice, different types of lasers are used [16]. The diode lasers are most often used because of their low price, satisfying efficiency, a high rate of modulation and a high longevity. The Helium-neon, Argon, Neodymium:yttrium-aluminium-garnet (Nd:YAG) and Erbium:yttrium-aluminium-garnet (Er:YAG) lasers are also used [17]. Exposing cells to low-level laser light in in vitro culture has a positive, bio-modulating result in stimulation of growth, proliferation as well as differentiation of cells into keratinocytes, fibroblasts, epidermal cells, myoblasts and osteoblasts [18, 19]. The data concerning the influence of laser light on stem/stromal cells are still limited and often contradictory, mainly because of the lack of standardization of the method caused by a constant oscillation about the choice of suitable parameters, such as wavelength, power density, dose, or time of exposure [20].
Aim
The aim of this study was to evaluate the influence of two lasers: Er:YAG (high power) and diode (low power) on the growth of hAT-MSCs in vitro.
Material and methods
The subcutaneous adipose tissue was obtained during the planned liposuction procedure from the abdominal area in the clinic of aesthetic medicine. The patients undergoing the procedure were female n = 6 (age range: 26–40 years; mean: 33 years) with BMI 22.5–30.9 kg/m2. The study protocol was approved by the Bioethics Committee of the Nicolaus Copernicus University in Torun, Collegium Medicum in Bydgoszcz (Approval Number: KB22/2017).
Adipose tissue-derived mesenchymal stromal cell isolation and culture
The isolation procedure of hAT-MSCs was carried out according to the protocol described previously by Zuk et al. with some modifications [21]. Lipoaspirate was extensively washed several times with Phosphate Buffered Saline (PBS, Corning, USA) containing 0.1 U/0.1 µg/ml penicillin/streptomycin (HyClone, USA) and 2.5 µg/ml amphotericin B (Corning, USA) and then was centrifuged at 400 xg/5 min. After removing the oily layer and the liquid layer located below the adipose layer, 20–30 g of tissue was weighed and then digested with 1 mg/ml of collagenase type P (Gibco, USA), in the ratio of 1 g of tissue/1 ml of collagenase, at 37°C for 10–15 min, mixed from time to time. The collagenase type P activity was then neutralized by adding an equal volume of Dulbecco’s Modified Eagle Medium (DMEM/Ham’s F-12, Corning, USA) supplemented with 10% Foetal Bovine Serum (FBS, Sigma-Aldrich, Germany), 0.1 U/0.1 µg/ml penicillin/streptomycin and 2.5 µg/ml amphotericin B. The dissociated tissue was filtered through a 100 µm pore filter to remove contaminants and centrifuged at 1200 xg/10 min. The obtained supernatant was removed and the remaining cells were suspended in the culture medium DMEM/Ham’s F-12 with the addition of 0.01 µg/ml basic Fibroblast Growth Factor (bFGF) and seeded at a density of 1.8–2.3 × 104 cells/cm2. The cultures were maintained at 37°C with 5% CO2.
The medium was changed every 2–3 days. After the cells reached 80-90% confluence, they were detached with 0.05% trypsin solution (Biomed-Lublin, Poland) – 0.5 mM EDTA (POCH, Poland) and subcultured at a density of 1–1.5 × 104 cells/cm2. Cell culture was carried out to the third passage.
Adipose tissue-derived mesenchymal stromal cell immunophenotypic characterization
To identify human MSCs, the International Society for Cell Therapy (ISCT) has recommended a panel of cell surface markers. AT-MSCs should be positive for CD44, CD73, CD90 and CD105, but negative for CD34, CD45, CD11b or CD14, CD19 and HLA-DR [22]. Therefore, the phenotype of hAT-MSCs cells was evaluated by flow cytometry using a commercially available kit for human Mesenchymal Stem Cells analysis (Human MSC Analysis Kit, BD Biosciences, USA).
For this purpose, 2.5 × 105 AT-MSCs, from the third passage, were washed twice with cold PBS solution and centrifuged at 4°C for 5 min. Then cells were suspended in Dulbecco’s Phosphate-Buffered Saline (DPBS, Corning, USA) with the addition of 2% Foetal Bovine Serum (FBS) and incubated in the dark for 30 min with antibodies. The cells were then washed twice in PBS, suspended in PBS and analysed with a flow cytometer (BD FACSCanto II, USA).
Adipose tissue-derived mesenchymal stromal cell multipotency analysis
The potential of AT-MSCs to differentiate into chondrogenic, adipogenic and osteogenic lines was performed using the following procedures. Cells from the third passage were cultured up to 70% confluence and then differentiated towards osteoblasts using StemPro™ Osteogenesis Differentiation Kit (Gibco, USA), chondrocytes using StemPro® Chondrogenesis Differentiation Kit (Gibco, USA) and towards adipocytes using Mesenchymal Adipogenesis Kit (Merck Millipore, USA), according to the manufacturer’s instructions. The morphological features of cultured cells were monitored every day using an inverted light microscope Leica DMi1 (Leica, Germany). After 14 days of chondrogenesis differentiation, the cells were stained with Alcian Blue solution (Sigma-Aldrich, Germany). The cells towards osteoblasts and adipocytes were differentiated for 21 days. The differentiation was confirmed by histological staining with Oil Red O (Merck Millipore, USA) for adipocytes and Alizarin Red Solution from Osteogenesis Quantitation Kit (Merck Millipore, USA) for osteogenesis. The results were examined under the inverted light microscope Leica DMi1 (Leica, Germany).
Laser irradiation of adipose tissue-derived mesenchymal stromal cell in vitro
AT-MSCs cells from the third passage were seeded at a density of 2 × 104/cm2 on 24-well culture plates (ThermoFisher Scientific Nunc, USA) leaving the empty wells next to the cells seeded wells to avoid cross-exposure of the cells. After 2 days the culture medium was replaced and the cells were exposed to high power Erbium:yttrium-aluminium-garnet laser (Er:YAG, SP Spectro, Fotona, Slovenia) and low power diode laser (SMARTMPRO, Lastronix, USA) according to the parameters shown in Table 1. Exposure was performed at room temperature. Laser irradiation was delivered perpendicularly, directly to the culture plates of 12 mm diameter using the same spot size to cover the entire surface of the plates evenly. The cells of the control group were under the same conditions of room temperature.
Cell viability assay
The MTT test was used to assess cell viability, which assesses the metabolic activity of mitochondrial dehydrogenase. The method is based on the reduction by mitochondrial dehydrogenase of water-soluble tetrazolium salt to insoluble formazan crystals. The ability to carry out this reduction is only possessed by living cells with normal metabolism and oxidative activity of mitochondria [23].
The MTT test was carried out 24 h after laser exposure of the cells. For this purpose, the culture medium was removed, cells were washed twice with PBS and incubated for 2 h in MTT solution (1 mg/ml) at 37°C in a 5% CO2 in the dark. Next, the supernatant was removed and dimethyl sulfoxide (DMSO, POCH, Poland) was added to dissolve formazan crystals. The absorbance was measured spectrophotometrically at 570 nm (test characteristic wavelength) and 655 nm (reference wavelength) using Varioscan LUX plate reader (ThermoFisher Scientific, USA). Unexposed cells were used as a control.
Statistical analysis
Statistical analysis was performed using SPSS Statistica version 24 (SPSS, USA) and evaluated using the Shapiro-Wilk non-parametric test, Levene’s severity test, ANOVA parametric single-variation analysis, parametric test and post-hoc tests. Differences at the significance level p < 0.05 were regarded as significant.
Results
Successful isolation and establishment of AT-MSC culture
Digestion of human adipose tissue by P-type collagenase allow for isolation of an average number of 2.7 × 105 ± 1 × 105 Stromal Vascular Fraction (SVF) cells. On the day after isolation the cells were attached to the cell culture flask surface and on the eighth or ninth day of culture they reached 80–90% confluence.
AT-MSCs from the third passage expressed MSC-specific markers
The results of cytometric analysis confirmed that the cells from the third passage had the phenotype typical for mesenchymal stem cells characterized by high expression of CD44, CD73, CD90, CD105 and low expression of CD34, CD11b, CD19, CD45, HLA-DR markers. Representative histograms are depicted (Figure 1 A).
Figure 1
A – Immunophenotype analysis. Detection of MSC surface markers expression (%) of CD44, CD90, CD73, CD105, CD45/34/11b/19/HLA-DR analysed by flow cytometry. Grey areas represent an antibody isotype control for background fluorescence and red areas show signal from MSC surface marker antibodies. Multilineage differentiation potential of AT-MSCs: B – adipogenic (inverted microscope: 10×), C – chondrogenic (10×), D – osteogenic differentiation (20×). Scale bars represent 200 µm
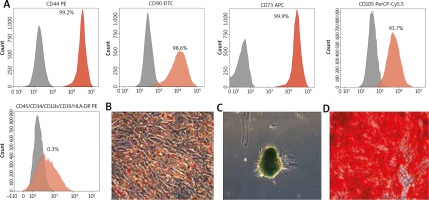
Adipose tissue-derived mesenchymal stromal cell multipotency analysis
Cells, which underwent morphological changes, demonstrate abundant amounts of intracellular lipid accumulation verified by Oil Red O staining (Figure 1 B). The chondrogenic potential was confirmed by formation of sulfated proteoglycans verified by Alcian blue (Figure 1 C). Osteocytes displayed accumulation of calcium deposits, formation of mineralized matrix nodules typical of osteogenic differentiation detected by Alizarin red staining (Figure 1 D).
Effects of laser irradiation on adipose tissue-derived mesenchymal stromal cell
This study demonstrated the stimulating effect of the Er:YAG laser irradiation on AT-MSCs growth at 5 Hz wave frequency, 0.1 J/cm2 (Figure 2 A) or 0.3 J/cm2 (Figure 2 B) dose and 4 s exposure time compared to the control (p < 0.05) (Figure 2 F). However higher 10 Hz wave frequency and 1.2 J/cm2 dose (Figure 2 C) led to a significant decrease in cell viability compared to the control (p < 0.05). AT-MSCs irradiation using Er:YAG laser with lower 5 Hz wave frequency gave a better biostimulative effect than higher 10 Hz wave frequency (Figures 3 A, B). Longer 4 s exposure time had also a better biostimulative effect on AT-MSCs growth than 2 s (2 s vs. 4 s) (Figures 3 C, D).
Figure 2
Morphology of AT-MSCs 24 h after irradiation with Er:YAG laser for 4 s at the frequency of 5 Hz, the dose of 0.1 J/cm2 (A), 0.3 J/cm2 (B), and at the frequency of 10 Hz, the dose of 1.2 J/cm2 (C) and with a diode laser, the dose of 1 J/cm2 (D), 4 J/cm2 (E) and control (F). Survival observations (10×, bar 200 µm)
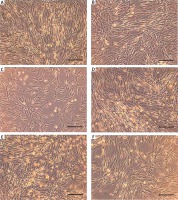
Figure 3
The viability of human adipose tissue-derived mesenchymal stem/stromal cells (hAT-MSCs) [%] 24 h after the exposure with Er:YAG laser light at the frequency of 5 Hz (A) and 10 Hz (B), the doses of 0.1; 0.3; 0.6; 0.9; 1.2 J/cm2 and the time of 2 s (C) and 4 s (D). The viability of AT-MSCs (%) 24 h after the exposure with diode laser light (E) using the doses of 1, 2, 4, 6, 8 J/cm2. MTT assay (*p < 0.05)
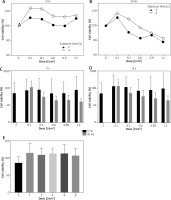
Diode laser irradiation led to stimulation of AT-MSCs growth in all analysed doses (Figure 3 E). The highest cell growth increases were observed when 1 (Figure 2 D), 2 or 4 (Figure 2 E) J/cm2 doses were used.
Discussion
Manufacturing of cellular therapy products requires substantial manipulation of the tissues and/or cells used as starting material. Obtaining a sufficiently large number of cells for transplantation in a short time is particularly important in the treatment of burns and post-myocardial infarction scars [24]. Growth of mammalian cells in vitro is relatively low compared to bacterial cells, which double their population every 30 min, whereas mammalian cells every 18–24 h. That is why it is so important to develop techniques to obtain the appropriate number of cells in a very short time. There are several ways to increase the rate of cell proliferation in vitro, such as adding growth factors to the culture medium and using other stimulants [10].
The low level laser therapy (LLLT) is successfully used in dermatology as an effective, safe and non-invasive method of treating difficult to heal wounds (e.g. diabetic foot [25, 26]), scars [1], burns [27] or other dermopathies, including the supportive therapy after a skin-graft [27, 28] and a mucosal transplantation [29]. Photobiostimulation therapy shows significant properties of cicatrising and regeneration of tissues; at the same time, it does not bring any overheating effects in neighbouring tissues. The process of cicatrising is accelerated by microvasculature stimulation, boosting the fibroblast proliferation [30, 31] and the synthesis of collagen [32, 33] as well as by stimulation of the immune system [34, 35].
So far, a lot of research concerning the use of cell therapies in treating difficult to heal wounds, burns or scars have been conducted [36]. Fibroblasts [37], keratinocytes [38–40], stromal vascular fraction cells sourced from adipose tissue [41–43], bone marrow stem cells [44] and blood cells were used in wound healing, both in clinical practice and in clinical research. However, further research are necessary in order to settle cell therapy as a standard way of dermatological treatment.
Photobiostimulation is a new concept to enable faster cell growth in vitro. It has been shown that stem/stromal cell proliferation can be increased by irradiating the cells with a low power laser. Laser irradiation of the place where the cells were implanted can stimulate their proliferation, increase the secretion of growth factors and thus increase the therapeutic effect [16]. An innovative, promising direction of research may also be the association of laser therapy with cell therapy [10].
Pouriran et al. combined the LLLT with the bone marrow stem cell therapy in treating difficult to heal wounds in patients with diabetes type I basing on the rat model. Applying cell therapy and laser therapy each alone as well as combining both methods were compared. The results of the experiment are promising, and combining the two methods in treating of difficult to heal wounds in the case of diabetes type I may bring measurable effects for the patient [45].
A similar experiment was conducted by Kouhkheil et al. with the difference that the wounds were infected with methicillin-resistant Staphylococcus aureus (MRSA). The results also showed a simulating influence of combining the LLLT and the bone marrow-derived mesenchymal stem cells (BM-MSCs) transplantation on regeneration of the area of the wound as well as on stimulation of immunological mechanisms by active eradicating of the infection [46].
Ali Nilforoushzadeh et al. conducted an experiment combining the LLLT with the autologous fibroblasts transplantation in treating third-degree burn. Ten diabetic patients with third-degree burns, qualified for skin-graft took part in the research. The experiment proved that the method of combining the LLLT with the autologous fibroblasts transplantation may be applied as an effective way of treating big wounds (burns), especially in diabetic patients [47].
The most popular sources of cells undergoing laser therapy were bone marrow and adipose tissue [16, 48]. In our experiment, the influence of laser therapy on the growth of hAT-MSCs in an in vitro culture was examined. The choice of the source of stromal cells resulted from the minimally invasive method of tissue acquisition, which was taken during liposuction procedures. It is not easy to compare the results of the experiment with those in the literature because different experimental conditions were applied: the type of cells, the type of the laser and its parameters. In the study, a high power laser – Erbium:yttrium-aluminium-garnet – was selected because of its common use in dermatology, for skin lesions destruction and skin rejuvenation, and a low power laser – diode, which was most often used by the authors of the works. The cells were exposed to a combination of frequencies, doses and exposure time. In the case of the Er:YAG laser light, the wavelength of 2940 nm, the frequency of 5 Hz and 10 Hz, doses of 0.1; 0.3; 0.6; 0.9; 1.2 J/cm2, and the time of exposure of 2 and 4 s were applied. In the case of the diode laser, the wavelength of 635 nm, quasi-continuous wave (QCW), doses of 1; 2; 4; 6; 8 J/cm2 and the time of exposure of 5, 10, 20, 30 and 40 s respectively were applied. The analysis of hAT-MSCs cells viability was performed 24 h after the exposure. Other authors irradiated cells to the light of lasers using the wavelength from 530 nm to 980 nm, and the doses from 0.03 to 45 J/cm2 [49–52]. The laser light of the wavelength of 600 nm and low doses were most often used. The frequency of the laser light was not mentioned in those studies. Most often the scientists performed analyses after 24, 53 and 72 h as well as after a few weeks. Tuby et al. showed that along with week lapse the cells increased their proliferation [24, 53]. Such an observation may not have a direct relatedness with exposure.
In the presented study, a significant stimulation of cells after Er:YAG laser exposure, 0.1 J/cm2 dose and 4 s exposure time and 0.3 J/cm2 dose and 4 s exposure time with frequency of 5 Hz, was shown in comparison with the control group (p < 0.05). At the frequency of 10 Hz and the dose of 1.2 J/cm2 a significant decrease of cells viability was observed in comparison to the control group (p < 0.05). It was noted that the doses 0.1; 0.3; 0.6; 0.9; 1.2 J/cm2 applied in Er:YAG laser brought better biostimulation results at the frequency of 5 Hz and the exposure time of 4 s in comparison to the frequency of 5 Hz and the exposure time of 2 s, or the frequency of 10 Hz and the exposure time of 2 or 4 s. It was proved that a high intensity laser stimulates the growth of hAT-MSCs. Not only is the length of the wave significant, but also a suitable combination of frequency, dose and exposure time. It was proved that lower frequency combined with smaller doses and longer exposure time have a positive influence on cells. The extended wavelength of 2940 nm and the high power of 20,000 mW are typical for the Er:YAG laser. Only a few papers discuss its positive influence on the growth of cells; in most of them, it is claimed that a high intensity laser will have a negative influence on cells, which certainly comes from the wrong assumptions. Pourzarandian et al. examined the results of exposure of fibroblasts with the Er:YAG laser using 1.68; 2.35; 3.37; 5 J/cm2 doses [50]. They showed that after 24 h there were no significant differences in cells growth, whereas after 72 h an increase in proliferation took place (except for the group exposed to the dose of 5 J/cm2). Pourzarandian et al. observations are contradictory to the results shown in the present research in which significant differences in cells growth comparing to the control group 24 h after the exposure were observed [50].
The doses of 1, 2, and 4 J/cm2 of the diode laser caused significant stimulation of cells growth (p < 0.05). The doses of 6 and 8 J/cm2 did not cause any statistically significant differences in relation to the control group. Hou et al. exposed BM-MSCs to the diode laser light at the wavelength of 635 nm and after 48 h an increase in proliferation was shown after applying the doses of 0.5, 1 and 2 J/cm2 [54]. Mvula et al. showed stimulation of AT-MSCs growth after an exposure to diode laser light of the wavelength of 635 nm, the dose of 5 J/cm2 and the exposure time of 15 min [7]. Wang et al., after exposure of MSCs to the laser light of the wavelength of 635 nm and the dose of 0.5 J/cm2, observed that the increase in proliferation was minimal after 24 h, and on the fourth day it was significant [52]. Karu proved that stimulation of cell proliferation depends on the dose, where low doses cause an increase in the rate of proliferation and other cell functions, whereas high doses have a negative influence on these processes. Contradictory results were shown by Cavalcanti et al. who observed in their experiment that higher doses of 6, 8 and 12 J/cm2 cause a significant increase in proliferation of BM-MSCs, and the lower doses of 1, 2 and 4 J/cm2 give worse results [55]. These results are also contradictory to those presented in this paper in which a stimulation of cells growth after applying lower doses and an increase in cells viability after an exposure with high doses were shown. There is a study using low-level laser (light) therapy (LLLT) applied to body contouring. The mechanism of LLLT-induced reduction of subcutaneous adipose tissue regrettably has not been elucidated and the suggested hypotheses are highly controversial. Patients’ adipose tissue (24 volunteers) was subjected to 650 nm LLLT therapy, each of them was subjected to six treatments with the LLLT device (Lipo Laser, Mimari, Poland) every 2 or 3 days over a 2-week period [2]. The research did not show a statistically significant reduction of abdominal subcutaneous adipose tissue after the LLLT therapy. Moreover, there were some side effects of using the laser, such as ulcers (in 2 patients). However, the paradoxical increase in thickness of the subcutaneous fat content, observed in 8 patients, is interesting, because of the stimulating effect of the lasers which are described here. The present research proved that a high intensity laser with correctly chosen parameters, at a low frequency and low doses, stimulates the growth of cells in vitro. Most researchers, starting with a wrong assumption that the high intense laser therapy always gives the result of reducing adipose tissue, do not evaluate the process of cells growth in an in vitro culture after exposure to high intensity laser light. The present paper is one of the first to evaluate the high intensity laser. The research conducted also confirmed the biostimulating properties of the low-level laser. Nevertheless, there is a need to repeat the research as well as to conduct further research extending the observation to 48 and 72 h.
Conclusions
The presented results indicated that both lasers, Er:YAG and diodes can be used to stimulate human Adipose-Derived Stromal/Stem Cells growth in vitro. The biostimulating effect of laser therapy on AT-MSCs may be used in the future in aesthetic dermatology in combined laser and cell therapy.








