Introduction
Silicone implants and expanders are commonly used in aesthetic and reconstructive breast surgery. Every year, plastic surgeons perform over 1.8 million breast augmentations globally, which makes this procedure the most common in the whole surgical esthetic panel [1]. Implant-based reconstructions have become a leading technique in both risk-reducing mastectomies and post-curative mastectomies, surpassing in number autologous breast reconstructions [2, 3]. The wide acceptance of silicon prostheses is based on the data supporting their health harmlessness. In the past, main concerns considered the risk of breast cancer in implant-exposed patients. It resulted from the data on the carcinogenetic role of polyurethane breakdown products in animal models, which undermined the safety of silicone polyurethane (PU) coated implants [4, 5]. These effects have never been proved in humans and until recently, breast implants were considered as causally unrelated to malignancies.
In 1997 Keech and Creech presented a case of a female patient after breast augmentation with an anaplastic large cell lymphoma (ALCL) mass in proximity to the saline-filled implant [6]. Since then, growing numbers of patients have proved the link between breast implants and ALCL in surrounding tissues. In 2016, the WHO classification separated breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) as a novel disease entity [7]. This malignancy, by definition, belongs to the non-Hodgkin lymphomas and results from the clonal proliferation of T-cells in peri-implant fluid or the fibrous capsule. Histologically BIA-ALCL represents large pleomorphic cells, immune-positive for CD30 T-cell antigens and negative for ALK proteins [8]. Prognosis in BIA-ALCL is favorable with 90% 5-year overall survival; however, in approximately 15% of cases it extends beyond the breast, invading regional lymph nodes or occasionally forming distant metastases [9]. This outcome is similar to the skin ALCL but is in contrast to the systemic ALK-negative ALCL [10, 11].
According to the American Society of Plastic Surgeons, 888 cases of BIA-ALCL were reported worldwide at the beginning of 2020, which makes BIA-ALCL a very uncommon disease compared to breast cancer and even to primary breast lymphomas [12, 13]. The estimated prevalence of ALCL in the general population ranges from 1 to 9 per 100 000 people [14]. The risk of ALCL located in the breast is much higher in women with implants compared to women with no implants or implant history. In 2018 de Boer et al. estimated the odds ratio of breast ALCL as 421.8 (95% CI: 52.6–3385.2; p < 0.001) for patients with breast implants [15]. Therefore, breast surgeons need information about the incidence of BIA-ALCL and practical guidance on how to diagnose and treat such patients. It is also patients’ right to be informed of the risk of BIA-ALCL while consenting for breast implant surgery [16, 17]. Likewise, patients exposed to rough surface implants expect surgeons’ advice, as, since 2018, pharmaceutical companies have been recalling the macro-textured implants due to the increased risk of BIA-ALCL [17, 18].
The multidisciplinary character and rarity of this disease require a national task force for data collection and advice service. Herein, we describe the first Polish multicenter case-series data of BIA-ALCL patients and present diagnostic and treatment recommendation for breast surgeons.
Material and methods
To collect the data of all BIA-ALCL patients in Poland, we addressed our search request directly to the members of the Polish Society of Surgical Oncology, the Polish Society of Plastic, Reconstructive and Aesthetic Surgery and the Extranodal Section of Polish Lymphoma Research Group, collecting BIA-ALCL cases in Poland. Additionally, we discussed this project with Polish hematopathologists during the Lymphoma Forum of Excellence – Pathology in Warsaw, on June 7th to 8th, 2019.
The study includes BIA-ALCL cases reported until March 2020. The survey contained questions about clinical data and types of implants. Stage of the disease was determined according to the TNM staging system for solid cancer and the Ann Arbor system for non-Hodgkin lymphomas [9, 19]. The follow-up data were obtained in March 2020 via phone calls.
The median time to BIA-ALCL occurrence was established from the implant insertion (the definitive implantation before diagnosis if multiple surgeries) to the first lymphoma symptoms. Median follow-up was assessed from the final surgical intervention for BIA-ALCL purposes to the last observation.
BIA-ALCL incidence was estimated in the female population age 30 and above, based on the data provided by Statistics Poland [20].
The Bioethics Committee of District Medical Council in Lodz has approved this study.
Results
We identified seven BIA-ALCL patients, diagnosed between July 2013 and November 2019. Median age at diagnosis was 46 (range: 30–64 years). Table I summarizes characteristics of the patients. The numbering of cases in the text refers to those in the table. Case 4 has previously been published [21].
Table I
Characteristics of BIA-ALCL patients diagnosed in Poland between July 2013 and November 2019
All the patients were exposed to textured implant surgery for aesthetic or reconstructive purposes (5 and 2 patients, respectively). Breast reconstructions were performed using multiple types of implants. The manufacturers of implants associated with BIA-ALCL (the last implant before the symptoms in numerous exposures) were Allergan (5 cases including McGhan), and Mentor and Silimed in 1 patient each.
One patient with multiple implant exposure (case 4) had direct-to-implant immediate reconstruction after prophylactic mastectomy, resulting in a sequence of unsatisfactory outcomes, capsular contracture and ruptures. These complications required multiple revisions using Nagor, Mentor, McGhan and Allergan implants. Finally, bilateral capsulectomies and implants’ replacement with Polytech were performed. The postoperative pathological assessment revealed BIA-ALCL in the unilateral breast, restricted to the capsule and peri-implant fluid. The patient refused any further treatment.
The second patient with multiple textured devices (case 6) underwent 2-stage breast reconstruction, in which both the expander and the implant had the same textured surface by Allergan. She was the only patient with breast cancer history in our series.
Median time from implant placement to the first BIA-ALCL symptoms was 65 months (range: 33–96 months). In 4 out of 7 patients, unilateral breast swelling was the single symptom at presentation and in one it was accompanied by breast pain. Altogether, breast pain was reported by 3 patients. The remaining loco-regional symptoms included palpable mass in the breast or axilla and capsular contracture. One patient presented systemic symptoms such as weakness, fever, dyspnea and lymphadenopathy.
In pre-treatment imaging workup, breast ultrasound (US) confirmed peri-implant seroma in all the patients. Additionally, in 3 cases, breast magnetic resonance imaging (MRI) was performed; in 3 cases, the images were misinterpreted. In 1 case, MRI described suspicious capsule thickening which proved to be inflammation in final pathology. In 2 patients implants assumed to have ruptured were intact at the surgery. Increased 18-fluorodeoxyglucose (FDG) uptake in the breast or axillary lymph nodes was detected in 3 cases out of 4 preoperative positron emission tomography/computed tomography (PET/CT) imaging.
Ultrasound-guided fine-needle aspiration (FNA) biopsy was performed in 4 patients, and in all the cases, cytology, supplemented by immunocytochemical staining, revealed BIA-ALCL cells. The flow cytometry analysis confirmed the diagnosis in 1 case. The patient with systemic adenopathy underwent surgical biopsy of the neck lymph nodes, which established the diagnosis. In 2 cases, BIA-ALCL was revealed incidentally in the routine histopathology of postoperative specimens.
Primary surgery was performed in all the patients, excluding the patient with systemic symptoms and advanced disease, who had received preoperative chemotherapy followed by high-dose chemotherapy supported by autologous stem cell transplantation. Capsulectomy with implant removal was performed in all the cases; in 5, it was a bilateral intervention, and in two a unilateral one. In two instances, immediate reimplantation was performed. Axillary node biopsy was complemented in 2 cases. One patient underwent re-excision of the breast wound due to the false-positive postoperative PET/CT scans. Adjuvant chemotherapy was administered in 1 patient.
In a median follow-up of 19 months (range: 5–81 months), there was no recurrence, and all the patients stayed alive. Figure 1 demonstrates an exemplary clinical presentation.
Figure 1
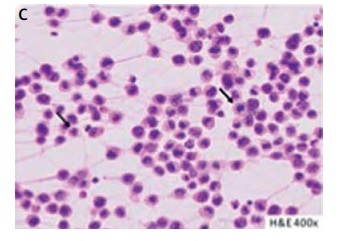
A patient with breast implant-associated anaplastic cell lymphoma (BIA-ALCL) (case 3). A thirty-year-old woman reported rapidly increasing swelling of the right breast (A). She negated other complaints. Six years earlier, she underwent bilateral augmentation with Silimed textured implants. Breast ultrasound revealed peri-implant seroma formation. Magnetic resonance imaging confirmed effusion layer 45 mm in width, covering the right implant; additionally, thickening of the fibrous capsule (white arrow) was noticed (B). Peri-implant layer on the left breast did not exceed 5 mm. Hematoxylin and eosin staining of effusion smear disclosed the presence of large lymphoid cells, with irregular nuclei (black arrows) (C). Numeous atypical cells showed intense positive CD30 immunocytochemical staining (black arrows) (D). Preoperative positron emission tomography scans were negative. The patient underwent bilateral implants removal with capsulectomies and breast reductions. The peri-implant fibrous capsules were sent intact for pathological assessment (E). The postoperative pathology did not disclose lymphoma invasions in either capsule, which settles the case to the stage IA according to the TNM classification and to IE in Ann Arbor staging for non-Hodgkin lymphomas [9, 17]. No adjuvant treatment was administrated. In 23 months of follow-up, the patient has remained recurrence-free; she has selected no other breast esthetics surgery (F)
Between 2013 and 2019, the annular incidence of BIA-ALCL among 100 000 women age 30 and above ranged between 0 and 0.021. Table II shows the detailed data.
Table II
Annular incidence of BIA-ALCL in Polish female population age 30 and above between 2013 and 2019. Denominators retrieved from Statistics Poland [16]. Data for 2019 carried forward from 2018 as it is still unavailable
[i] BIA-ALCL – breast implant-associated anaplastic large cell lymphoma [18].
Discussion
Current studies indicate that the textured surface of the implant and bacterial contamination contribute to BIA-ALCL pathogenesis [22]. Compared to the smooth implants, the rough outer shell of the implants gives beneficial conditions for bacterial attachment and growth, which triggers the host chronic inflammatory response [23]. Consequently, the sustained T-cell activation may promote aberrant clones, leading to symptomatic BIA-ALCL [24].
The study of Loch-Wilkinson et al. confirmed that the higher surface area textured implants of Biocell (Allergan) and polyurethane (Silimed) markedly increase the risk of BIA-ALCL [25]. Similarly, in our study, 6 of 7 patients were exposed to textured implants, which corresponds with the hypothesis of a role of such a surface in BIA-ALCL genesis. Presumably, the decision to recall the aggressive-textured implants from the world markets, including the EU, will cause this disease to become even more scarce [18, 26]. Incorporating the aseptic procedure, e.g. a 14-point plan for implant placement, and further genetic research to select high-risk patients would fall within this optimistic scenario [27].
Clinical presentation of BIA-ALCL has usually developed within 7 to 10 years from implantation as unilateral breast swelling caused by spontaneous, non-inflammatory peri-prosthetic effusion [9, 22, 23]. This so-called “late and cold seroma” is not pathognomonic for BIA-ALCL, as it might result from subclinical infections or trauma [8]. Moreover, a thin liquid coating of the implant whose volume did not exceed 5–10 ml is typical and does not require further tests [9]. In our series, onset of the disease was reported earlier, in a median time of implant exposure just under 5.5 years. Besides breast enlargement, breast pain was also often a reported symptom in our series. Palpable breast mass, the second frequent symptom of BIA-ALCL described in the literature, was observed only in a stage IV patient [8, 9, 22, 23].
There are several imaging techniques in detection of a peri-implant effusion or mass, including breast ultrasound (US), mammography, MRI, chest computed tomography (CT) and PET/CT [9, 22, 23, 28, 29]. In a retrospective study Adrada et al. assessed the effectiveness of these modalities in detection of effusion and mass in 44 BIA-ALCL patients [28]. Breast US and MRI achieve the highest sensitivity for effusion disclosure, exceeding 80%. Except for PET/CT, no other technique gained over 50% sensitivity in mass detection. In our study, breast US revealed excessive peri-implant effusion in all the cases. Breast MRI was performed in 3 patients but their results were misleading regarding the capsule involvement and implant ruptures. It may show that breast MRI assessment in BIA-ALCL suspicion might be challenging, which makes it an addition to the routine US rather than an alternative. Still, a preoperative MRI serves as a tool to exclude breast cancer before the initiation of BIA-ALCL treatment.
None of our patients had preoperative mammography, which might be motivated by its limited role in augmented or reconstructed breasts [30]. Although mammography is a standard tool in breast cancer screening programs, it is insufficient to distinguish effusion from the mass [28, 31]. Similarly, CT chest scans were not made during preoperative workups in our series, as this modality does not belong to the routine breast imaging.
PET/CT imaging is the method of choice in the assessment of the disease staging, treatment response and follow-up in FDG avid lymphomas [32, 33]. Notably, in BIA-ALCL, timing of PET/CT remains debatable, due to the increased risk of post-biopsy or post-surgery false-positive results [9, 33, 34]. In our study, two of three preoperative PET/CT overestimated the nodes staging. Additionally, in 1 case, early postoperative PET/CT showed increased FDG uptake in the pectoralis major muscle, which led to the re-excision of the breast tissues. In this case, histopathological examination did not confirm residual lymphoma, which extends the pool of PET/CT false-positive results in our series. Therefore, we conclude that preoperative PET/CT imaging should be limited to patients with locally advanced or systemic symptoms of BIA-ALCL, who would benefit from primary chemotherapy. We recommend postoperative PET/CT imaging in all patients, after complete wound healing.
Clonal T-cells usually occur in the peri-implant effusion. Therefore, BIA-ALCL diagnosis is based on cytology with immunostaining [8]. Direct smears are likely to be insufficient for definitive diagnosis; consequently, it is necessary to aspirate about 20 to 50 ml of effusion and send it fresh to a laboratory. In our series, in 2 cases, in which effusion was collected preoperatively, this approach enabled BIA-ALCL to be diagnosed. In 1 case, fluid was sent for flow cytometry, which confirmed the initial diagnosis. As T-cell clonality evaluation clarifies T-cell origin of BIA-ALCL, additional molecular tests are particularly required. In uncertain morphological and immunophenotypic features, e.g. in a lymphocyte-rich seroma with few (< 10%) atypical CD30+ cells, flow cytometry- or polymerase chain reaction (PCR)-based methods (e.g., gene scan analysis) allowed evaluation of clonal rearrangement [35]. Besides the benefits of molecular facilities, we share the opinion that for timely diagnosis and costs, they should be limited to the cases of equivocal cytology and immunostaining [36].
All the patients in our study underwent capsulectomy with implant removal, which is a standard treatment of BIA-ALCL [9, 22, 25, 34–39]. In most cases, the procedures were bilateral, which was dictated by esthetic reasons to maintain the symmetry or by patients’ and surgeons’ concerns. Still, the risk of asymptomatic BIA-ALCL in the contralateral breast is low; according to Clemens et al., it is below 5% [38]. Little is known about breast reconstruction in BIA-ALCL patients. We believe immediate breast reconstruction may be justified only in early-stage patients, who are highly determined to undergo such an approach. If a patient expects delayed breast reconstruction, exclusion of lymphoma recurrence is mandatory. Regardless of the timing of breast restoration, autologous tissue or smooth implants should be used rather than textured devices [39].
The differential diagnosis of BIA-ALCL includes systemic ALK-negative ALCL with secondary involvement of the breast. History of breast implantation is crucial. In our study, 1 patient with systemic BIA-ALCL received primary intensive chemotherapy followed by high-dose chemotherapy supported by autologous stem cell transplantation. Afterwards, she underwent surgical bilateral capsulectomies with explantations. This case emphasizes that a patient with breast implants and ALK-negative ALCL diagnosis should have a thorough breast examination, including ultrasound or MRI. Such an approach allows diagnosis of an advanced stage of BIA-ALCL, which, in addition to chemotherapy, requires surgical treatment.
There are several algorithms of BIA-ALCL treatment, which depict an optimal way from diagnosis, via the treatment to the follow-up [9, 22, 34–38, 40, 41]. They are systematically updated and may differ depending on the country-specific healthcare systems. As a consensus of the Polish task force for BIA-ALCL, we proposed 10-point guidelines for the surgeons who use breast implants in esthetics and reconstructive indications (Table III).
Table III
Practical guidelines for breast surgeons for BIA-ALCL diagnosis and management
|
The main disadvantage of our study is that we did not assess the risk of BIA-ALCL in Polish population. Lack of reliable data of the number of women with breast implants in Poland was the reason. The national commercial law ensures confidentiality of the sales data, including the expanders and implants market. Furthermore, our sighting study showed insufficiency of the questionnaire survey on plastic surgery and surgical oncology centers. For these reasons, the incidence of BIA-ALCL in the Polish female population exposed to the breast implant remains unknown. Instead, we estimated the annular incidence of BIA-ALCL in the total Polish female population, excluding women under 30, whom breast implant surgery seldom concerns. In this selective group, the rate was under 0.021/100 000/year. It fulfilled the criteria of rare cancer, defined as malignancies with incidence below 6/100 000/year according to RARECARE – the project Surveillance of Rare Cancers in Europe [42, 43]. Rare cancers are a significant public health problem, as in total, their various types represent 10–24% of all tumors diagnosed in Europe – according to European Society for Clinical Oncology [44]. Although the RARECARE registry has not included BIA-ALCL, the risk of inadequate diagnosis and treatment (both under- and overtreatment) also concerns this entity. Moreover, if we consider BIA-ALCL as an iatrogenic disease, any experience of patient care as an understanding of pathogenesis is valuable to avoid such jeopardy in future. It justifies all efforts to create national BIA-ALCL registries and task forces, and boosts international research collaboration.
In conclusion, both breast augmentation and postmastectomy reconstruction are procedures improving quality of life. Oncological safety is crucial in this context. Our study confirmed that in the Polish population, BIA-ALCL remains scarce and has a generally excellent prognosis. We can foresee that due to the withdrawal of roughly textured implants from the market, and thereby exclusion of likely the most potent etiologic factor, the incidence of BIA-ALCL will decrease with time. However, it does not exempt a breast surgeon from informing patients of the risk of BIA-ALCL and further vigilance during their follow-up. We believe practical guidelines proposed in our paper will be a helpful tool to manage BIA-ALCL patients fast and safely.