Dear Editor,
Clostridioides difficile infection (CDI) has become a serious medical and epidemiological problem with a marked increase in incidence and severity [1]. CDI is responsible for 15–25% of all anti-biotic-associated diarrhea cases, with a considerable increase in the 21st century [2]. Recommended first-line therapy for CDI involves oral vancomycin [1]. Oxygen therapy might be a variant of etiological therapy for anaerobic infection, including CDI [3]. Here we present a case of successful treatment of clostridial colitis in a child by enteral oxygen administration (state scientific project “Investigation of gastrointestinal tract nondigestive functions and the role of enteral oxygenation”). Ethical approval for the clinical usage of enteral oxygen administration was provided by the local ethical committee. Informed consent of the patient’s mother was obtained for enteral oxygen therapy.
A child was delivered by Caesarean section at 32 weeks of gestation. The mother was infected with herpes simplex virus and cytomegalovirus infections. Moreover, the pregnancy was exacerbated by chronic placental insufficiency, fetal delay syndrome, and moderate intranatal asphyxia. The child’s condition was critical from birth because of prematurity and respiratory failure, and he received non-invasive respiratory support (nasal positive airway pressure).
From the third day of life, due to the volvulus intestinal and recurrent ischemia of the small intestine and stomach, several surgical interventions were performed: resection of a necrotized and perforated section of the small intestine 35 cm long, application of a single-trunk eunostoma, suturing the perforation of the anterior stomach wall. As a result, the total length of the remaining small intestine was 25 cm. The child’s condition remained severe due to sepsis and multiple organ failure. He was receiving long-term antimicrobial therapy.
At the age of 20 days, the patient was transferred to the Almazov National Medical Research Center, and four days later was operated on for bowel obstruction with jejunostomy.
At the age of 1.5 months, the patient underwent reconstructive surgery – end-to-end jejuno-ileo-anastomosis; an unloading appendicostomy was applied. The volume of enteral feeding was limited due to the development of short-bowel syndrome, and the patient received partial parenteral nutrition.
At the age of 3 months and 7 days, excision of gastric ulcer and gastrography due to recurrent bleeding were performed. The patient received a repeated course of antimicrobial therapy.
At the age of 4 months and 8 days, the patient had stool up to 10 times a day with large water losses. Three days later, high titers of Klebsiella pneumoniae and Clostridioides difficile toxins A and B in feces were detected. Enteral nutrition was discontinued, and oral administration of vancomycin was started. A nasointestinal feeding tube (ConvaTec, Nitra, Slovakia) was introduced transrectally to the splenic flexure of the colon, through which 100% oxygen was delivered at a rate of 2–5 mL h-1 using a 50 mL syringe pump (B. Braun, Hessen, Germany). The total volume of enteral oxygen was about 50 mL per day.
During the next five days, the patient’s condition was stable: he did not have a fever and gained 200 grams.
Clostridioides difficile toxins A and B test results were negative. The stool normalized, and the volume of enteral feeding was gradually brought up to the previous level – 40% of the desired volume.
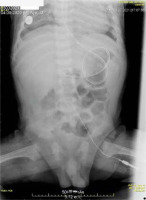
At the age of 5.5 months, the patient had a relapse of clostridial colitis (Clostridioides difficile toxins A and B tests were positive). Clinically, at the very beginning, diarrhea with stool up to 8 times a day was noted. The maximum body temperature was 37.6°C. The patient’s condition remained stable; however, enteral nutrition was cancelled, and enteral oxygen therapy was started again. Oxygen (100%) was delivered at a rate of 3–5 mL h-1 using the same syringe pump as described above (the ConvaTec nasointestinal tube, placed at the first (superior) part of the duodenum; Figure 1). Considering the bactericidal effect of oxygen against obligate anaerobic bacteria, enteral oxygenation was carried out first as a monotherapy for the episode of pseudomembranous colitis; antimicrobial therapy (vancomycin) was not prescribed.
FIGURE 1
Abdominal cavity X-ray demonstrating nasointestinal tube performing enteral oxygenation in a child with Clostridioides difficile colitis
Two days after the initiation of enteral oxygen therapy Clostridioides difficile toxins A and B test results were negative, stool frequency and consistency normalized. Along with continuous enteral oxygen therapy, enteral feeding was resumed and gradually returned to its previous volume. Repeated tests for Clostridioides difficile toxins were negative.
Finally, enteral oxygen therapy was stopped (duration of enteral oxygen treatment was 11 days), and the intestinal tube was removed. The patient’s condition was stable, and he received the previous volume of enteral and partial parenteral nutrition due to short-bowel syndrome.
During the next five days, repeated tests for Clostridioides difficile toxins A and B were negative. So, CDI relapse had been cured without vancomycin use.
Enteral oxygenation has been used in experimental and clinical settings [4–8]. In particular, oxygen has been used as adjunctive therapy for clostridial myonecrosis and necrotizing fasciitis [9]. In 1998, Katsuro et al. [10] described a case of successful treatment of toxic megacolon with hyperbaric oxygenation (HBO). In the same year, Schechter [11] suggested the need to test the hypothesis of the possibility of using HBO for the treatment of toxic megacolon, which is usually the result of CDI according to Sheth et al. [12].
The most desirable goal of enteral oxygen therapy, however, was (and is) to increase systemic oxygenation. In 2015, we published the results of our clinical investigation describing arterial oxygenation improvement following enteral oxygen therapy [7]. Recently, similar data were obtained by Okabe et al. [13] in an experimental setting, where oxygenated perfluorocarbon was administered rectally.
Considering enteral oxygen therapy for CDI treatment, there are some questions that need to be addressed. First, how much oxygen should be administered intestinally to reach its bactericidal concentration in the colon region where anaerobic bacteria reside predominantly? Second, which way of oxygen delivery is preferable: nasointestinal or intra-rectal? We suggest that the nasointestinal route is better than others. Because of diarrhea and high gastrointestinal movement, oxygen, insufflated into the small intestine, would not be absorbed completely there and could reach the large intestine. Moreover, the nasointestinal route is preferable since the oxygen delivery tube would not be pushed out with feces. Further investigations will be focused on clarifying these issues.
We believe that the described clinical case represents the first experience of etiologic therapy of clostridial colitis by enteral administration of small doses of oxygen.




