Introduction
Behçet’s disease (BD) is a multisystemic disorder, characterized as triple symptoms disease with recurrent genital ulcer, oral aphthous, uveitis, and even more serious complications including blindness and intestinal perforations [1]. BD is generally perceived as a kind of autoimmune disease, and its identification as of autoimmune origin could be significant for its diagnosis and treatment [2]. Researchers have debated whether there is a potential autoimmune basis for BD, but autoantigens related to BD and their pathogenesis had not been elucidated clearly when compared with other classic autoimmune diseases such as systemic lupus erythematosus (SLE) [3, 4].
Although researchers had made great progress to understand the BD pathology, but the exact philosophy is only partially understood. For example, epitopes on cell membrane proteins may cross-react with antibodies and become BD autoantigens, as we investigated some important BD markers including heat shock proteins (HSP 27), prohibitin (rhPHB), electron transfer flavoprotein subunit beta (ETFB), and annexin A2 [5-8]. These findings not only revealed the vasculitis property of BD, but also showed the pathogenesis of BD as it might originate from various pathogenic infections.
Similarly, some nuclear proteins have been identified and described as autoantigen like hnRNP family (hnRNP A1 and hnRNP A2) [9]. In our previous study, HUVEC and HEK293 cells showed immunofluorescence and nuclear staining pattern of BD sera [5-8], which looks very similar to the target protein staining pattern [10-12].
NuMA is an important nuclear protein, which is disseminated in the peripheral centrosomal region to each spindle pole during the process of mitosis [13]. The characterization of reactive autoantibodies in immunofluorescence patterns with NuMA has been defined in interphase stage of cells, in which it looks like a fine, freckled nuclear staining with negative nucleoli, whereas mitotic cells have bright staining on spindle poles [14]. On the basis of sequence analysis, some key antigenic epitopes [15, 16] were considered to be mainly located in the C-terminal of NuMA. Therefore, the aim of this study was to prove whether NuMA is the target antigen of BD and the existence of antibody against C-NuMA in the blood circulation may cause BD pathology.
Material and methods
Collection of samples
Serum samples were collected from 32 BD patients (average age, 40 years; range, 18-80 years; 12 males,20 females), who fulfilled the international BD diagnostic criteria [17]. The control sera from 32 healthy donors (average age, 25 years; range, 21-32 years; 25 females,7 males) and 32 patients with SLE (average age, 31 years; range, 16-54 years, 26 females, 6 males) were selected. Ethically approved samples were provided by the Chinese PLA General Hospital. Samples were taken from consenting patients, and stored at -80 °C.
Indirect immunofluorescence assay
The HEK293 cells were cultured in DMEM medium containing 10% fetal bovine serum (HyClone, UT), using a procedure described before [18]. In brief, the cultured cells were placed on microscopic slides with cover slip, with freshly prepared 4% paraformaldehyde fixative. The coated slides were washed with water at least three times. Then, 10 BD patients’ sera (1:20) in PBS were added, and the slides were incubated for 1 hour at 37°C temperature. After extensive washing, the slides were incubated with fluorescein isothiocyanate-conjugated antibody (goat-anti human IgG) (ImmunoHunt, Beijing, China), which was diluted 1:200 in PBS at 37°C temperature for 1 hour. Slides were adjusted for examination under fluorescence microscope (AMG, Bothell, WA, USA).
Plasmid construction, expression, and purification of NuMA C-terminal peptide
Based on our standard lab procedures described in detail before [5], the cDNA encoding the open reading frames of C-terminal peptide (C-NuMA, amino acid residues 1522-2115) was achieved by RT-PCR, using extracted total RNA from HEK293 cells as a template. Then, the cDNA of C-NuMA was amplified using adopter primers with the EcoRI and HindIII recognition sequences. The particular sequences of primers were as follows:
5’-GAA TTC ATG GGA AGA ACT GAG-3’ (forward primer of C-NuMA, EcoRI restriction site);
5’-AAG CTT GTG CTT TGC CTT GC-3’ (Reverse primer of C-NuMA, HindIII restriction site).
The obtained PCR product was cloned into pET-28a (+) vector (prokaryotic expression vector preserved by our lab), and were over-expressed in E.coli BL21 (DE3). The expression products were purified by high affinity Ni-charged resin (GenScript, NJ, China) and then stored at -80°C freezer for further analysis.
Mass spectrometry
The procedure of mass spectrometry was described before [19]. In brief, 2 µg purified recombinant human C-NuMA protein was denatured (100°C for 10 minutes) and analyzed by SDS-PAGE. The excised gel was destained with 50% acetonitrile and 25 mM NH4HCO3, and dried in a vacuum centrifuge. Then, the gel pieces were incubated with 25 mM NH4HCO3 (adding 10 mM dithiothreitol) for 2 hours at 37°C temperature. Subsequently, the gel pieces were added into 25 mM NH4HCO3 (adding 55 mM iodoacetamide) to incubate for 45 minutes at room temperature in the dark. Next, gel pieces were washed with 25 mM NH4HCO3 for 10 minutes and dried in a vacuum concentrator (Savant Instruments, NY, USA). Finally, the gel was digested in 20 µL 0.05 M NH4HCO3 buffer containing trypsin (Sigma-Aldrich, MO, USA) at 37°C overnight. The peptides were acquired using the AB4700 Proteomics Analyzer (Applied Biosystems, MA, USA). The Mascot search engine was used for data analysis (Matrix Sciences, London, UK).
Western blotting
The procedure of Western blot was described previously [20]. The purified recombinant proteins of C-NuMA were separated by SDS-PAGE and transferred to the polyvinylidene difluoride (PVDF) membrane. Blocked the membranes with 5% non-fat milk in PBS at 37°C temperature for 1 hours, the membranes were then incubated with 10 sera from BD patients (same group to immunofluorescence detection) and 10 healthy controls (1:1000 dilution) at 4°C temperature, overnight. After three times washing with 1% PBST buffer, the unbounded antibodies were removed. Then membranes were immersed with conjugated HRP-goat anti-human IgG (1:6000 dilutions, ImmunoHunt, Beijing, China) for 1 hour at 37°C temperature. Then, the membranes were detected by chemiluminescence kit (Applygen, Beijing, China).
Immunoprecipitation
The immunoprecipitation assay was performed as per our recently published study [12]. The C-NuMA was incubated at 4°C temperature for overnight, with equal volume of positive BD sera, which was used in the Western blotting. Then, A-sepharose beads (Sigma, MO, USA) were added and kept at 4°C for overnight. To obtain the immune complexes (antigen-IgG complexes), the mixture were centrifuged for 2 minutes at 5,000 rpm, and the supernatants were separated into another centrifuge tube. Then, the immune complexes were washed three times with 200 µL (0.1% PBST). The immune complexes and the supernatants were both detected by SDS-PAGE.
ELISA
ELISA procedure was performed as previously defined [21]. The recombinant protein C-NuMA (100 ng/mL) was coated on microtiter plate with 0.05 M carbonic buffer for overnight at 4°C. Subsequently, 100 µL goat sera 10% was added as a blocking material and incubated the plate for 2 hours at 37°C. The material was dispensed and the plates were patted with paper towel. Then, the 100 µL sera of BD, SLE, and HC were added in dilution buffer (1 : 100), and the plate was left in incubator at 37°C temperature for 1 hour. Then, the liquid material was removed and the plate was washed with 0.1% PBST buffer. Secondary antibody HRP-conjugated goat anti-human IgG (1 : 20000 dilutions) was added to each well for 1 hour at 37°C. Then, TMB substrate 50 µL was added and the plate was kept in a dark place at room temperature for 10 min, which produced blue color. Finally, the stop solution was added and the antibody titer was measured by ELISA reader (Tecan, Hombrechtikon, Switzerland) at 450/ 620 nm.
Statistical analysis
Statistically, results were measured by Fisher’s exact test with prism version 5 (GraphPad, La Jolla, CA, USA) to estimate the prevalence of autoantibodies in BD patients and healthy control (HC) samples. ELISA results were analyzed by t test using SPSS software (version 17, IL, USA). P < 0.05 was considered significant. The definition of critical point was a number with a higher value than that of the healthy controls (mean, +2 SD).
Results
Indirect immunofluorescence assay
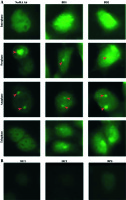
The HEK293 cells were used to screen the BD sera and autoantigen was verified at cell level. Cells were incubated with anti-NuMA antibody, which indicated a classic pattern of NuMA protein. The two BD sera matched with the pattern of antibody staining (Fig. 1).
Fig. 1
Immunofluorescent pattern of samples containing anti-NuMA antibody and BD sera. A) HEK293 cells stained with BD serum and antibody to NuMA show the same pattern in various stages of division, an intense positive reactions in HEK293 cells with BD sera. B) HEK293 cells were incubated with HC serum and PBS served as a control
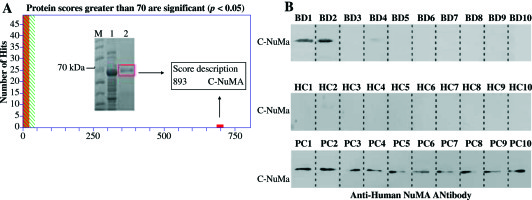
Identification and verification of NuMA autoantigen
The purified recombinant protein was identified by MALDI-TOF/TOF mass spectrometry. Peptide mass finger printing detail and amino acid sequences showed a protein score as 893, the coverage of the amino acid sequences were 22% of NuMA (UniProtKB/Swiss-Prot. number Q14980), and the credible peptides were present in the C-terminal of NuMA sequences (Fig. 2A). To determine the activity of the recombinant proteins, Western blot analysis was performed with sera samples from BD patients and healthy people (based on staining of the mitotic spindles). The recombinant protein could be significantly recognized by sera from BD patient, but not by healthy donor (Fig. 2B).
Fig. 2
Analysis of purified recombinant protein by mass spectrometry and Western blot. A) NuMA protein was overexpressed in E. coli BL21 after IPTG-induced expression (band 1) and purified using Ni-NTA (band 2). Then, the amino acid sequences of the purified band were identified by MALDI-TOF-TOF mass spectrometry. B) The Western blot analysis of NuMA probed with 10 BD patients sera and 10 HC as a result, 2 BD patients sera reacted positive, but no HC sera. The recombinant purified protein reacts with anti-NuMA antibody as positive control (bottom row)
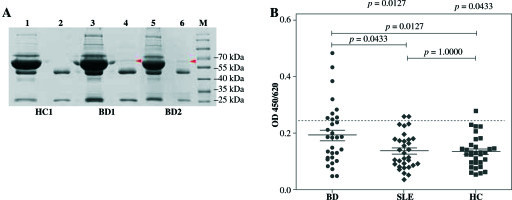
The prevalence of antibody against C-NuMA
Immunoprecipitation was performed to verify the results of Western blot (Fig. 3A). Immunoprecipitates confirmed the presence of 67 kDa band indicating that C-NuMA was indeed an autoantigen of BD. ELISA evaluations were performed with the recombinant C-NuMA protein, which revealed autoantibodies in 9 of 32 patients with BD (28%), 2 of 32 patients with SLE (6%), and 1 of 32 with HC (3%), based on the cut-off value and mean +2 SD of HC (Fig. 3B). It was observed that the anti-NuMA antibody had a significantly higher prevalence in BD than SLE (p< 0.05) or HC (p< 0.05), which further confirms that NuMA has a immunogenic worth in BD pathogenesis.
Fig. 3
Prevalence of anti-NuMA antibodies in patients with BD. A) Immunoprecipitation with human recombinant NuMA protein with sera from BD patients was also performed to verify whether it was an autoantigen of BD. The C-Nu- MA protein band was clearly present in the immune complexes (band 4, band 6), but not in supernatant (band 3, band 5) and neither in HC (band 1, band 2). B) Sera anti-NuMA antibodies were detected by ELISA in 9 of 32 BD patients, 2 of 32 SLE patients, and 1 of 32 HC. Data was analyzed as mean ±SD
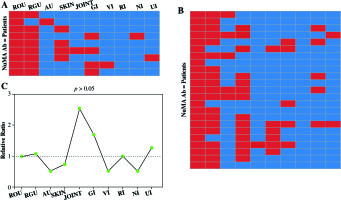
The clinical characteristics in BD patients
Out of 32 BD subjects, 9 showed positive reactions with recombinant C-NuMA proteins and 23 patients showed negative reaction. In BD patients with positive reactivity for recombinant human C-NuMA, symptoms were observed in patients as recurrent oral ulcers in 9 patients (100%), recurrent genital ulcers in 8 cases (89%), skin lesions in 4 patients (44%), gastrointestinal involvement in 4 cases (44%), and only one subject with anterior uveitis, joint involvement, and vascular involvement was observed, respectively. No major clinical differences appeared when compared to 9 anti-NuMA antibody-positive with 23 negative subjects. Details are summarized in Figure 4.
Fig. 4
Clinical significance of BD patients. A) Nine BD patients who were anti-NuMA antibody-positive were analyzed to decide whether NuMA is related to clinical symptoms. Every rectangle accounts for a patient. The red color states that the patient is involved in corresponding organ damage. B) The clinical information of 23 anti-NuMA antibody-negative BD patient samples. C) The comparison of the clinical information between sera anti-NuMA antibody-positive and negative groups, the ratio was calculated by method of positive group (Numberclinical+/Numberclinical-)/ negative group (Numberclinical+/Numberclinical-) (ROU – recurrent oral ulcers, RGU – recurrent genital ulcers, SKIN – skin lesions; AU – anterior uveitis, V – vascular involvement, GI – gastrointestinal involvement, NI – nervous system involvement; JOINT – joint involvement, RI – respiratory system, UI – urinary system)
Discussion
NuMA is nuclear protein and plays an important role in mitotic spindle formation. It consists of globular head, tail domains, and a central rod domain separating the head domains from tail domains as a coiled-coil spacer [22]. As it contains a nuclear targeting sequence, the site for binding to the mitotic spindle is responsible for nuclear reformation[23]. It has been proposed that the C-terminus of NuMA contains binding site for tubulin (Fig. 5A), and its involvement in BD pathology was reported [24]. NuMA has a strong affinity and directly binds to tubulin [25]. BepiPred linear epitope prediction analysis showed [26] twenty-eight epitopes that were predicted for C-terminus of NuMA. The average score of C-NuMA (1522-2115) was higher than the remaining peptide of NuMA (1-1521), as shown in (Fig. 5B), indicating that some antigenic epitopes are located on the C-terminal of NuMA.
Fig. 5
A) Schematic representation of the secondary structure of full-length NuMA. The position of tubulin is given on the full-length sequence. B) Antigenic epitopes prediction result of NuMA. The yellow color indicate feasible epitopes, but the green not according to IEDB Analysis Resource (http://tools.immuneepitope.org/main/). The threshold of predicted residue scores is 0.399
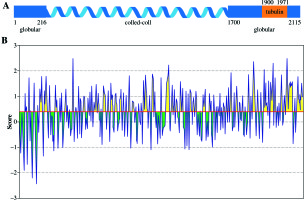
As cell surface proteins carry epitopes, which can cross-react with antibodies, similarly, several nuclear proteins have been detected at cell surface, and in most cases they were called as autoantigens [27]. Since the existence of autoantibodies is a matter of discussion, autoantibodies to the NuMA protein were initially reported in a variety of autoimmune conditions including Söjgren’s syndrome, polyarthritis, and SLE [28]. NuMA as antigen was confirmed by immunofluorescent staining of mitotic spindle apparatus and immunoprecipitation methods [29, 30].In this study, we used purified recombinant human C-terminal domains of NuMA to analyze the sera levels of autoantibodies. It was observed that 9 patients exhibited a positive reaction, among the 32 BD patients. Evidences from previous studies showed that NuMA is one of target proteins during programmed cell death [31]. The injection of NuMA antibody to interval and progenitor cells can lead to disappearance of the mitotic apparatus [32].
Conclusions
Keeping in view the above facts and findings, it is suggested that the existence of NuMA antibody in BD patients may lead to the same results targeted as cell apoptosis. Although NuMA is not associated to a defined autoimmune pathology, the presence of high titers circulating antibodies against C-NuMA may be a potential target antigen in Han Chinese BD patients.


