In December 2019, several cases of pneumonia with unknown cause were reported in the city of Wuhan, China. Subsequently, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified as the causative organism, and the disease was termed Coronavirus disease 2019 (COVID-19). The clinical features in most of those affected included fever and dry cough, but many patients also developed respiratory failure requiring ventilatory support, overloading intensive care units (ICUs) and health systems in a large number of countries.
The literature regarding the management of acute respiratory distress syndrome (ARDS) caused by SARS-CoV-2 is scarce, limited to a few editorials and some observational studies [1–4]. Some authors have suggested that COVID-19 infection would give rise to various ARDS phenotypes (typical with low compliance or atypical with relatively preserved pulmonary compliance), which would require a different ventilatory management adapted to each one [1, 2, 5]. However, other authors have not found similar results and argue that ARDS caused by COVID-19 has similar characteristics to ARDS of another aetiology [3, 6].
Prone positioning may be useful as an early rescue manoeuvre for severe hypoxaemia in patients with ARDS [7–10]. However, there are not many studies that have investigated the response to the prone position in intubated COVID-19 patients with ARDS [4].
We describe the clinical features, respiratory pathophysiology, and clinical outcomes of COVID-19 patients with respiratory failure treated with invasive mechanical ventilation in the prone position in a single tertiary care hospital in Spain.
METHODS
Study design
This is a retrospective study that enrolled patients with ARDS due to COVID-19 admitted to the ICUs of Salamanca University Hospital, Spain. The study was approved by the Institutional Review Board of our hospital (code number: PI 2020 05 487). The requirement of informed consent was waived.
Study population and data collection
Data were collected from patients’ electronic medical records. The study included all COVID-19 patients admitted in 2 ICUs in our hospital between March and April 2020, who met the following criteria: ≥ 18 years old, acute ARDS as defined by the Berlin criteria, intubated, and mechanically ventilated.
Hospital management recommendations for treating ARDS patients included: tidal volume (TV) targeting 6 mL kg-1 of predicted body weight, and positive end-expiratory pressure (PEEP) adjusted by the treating physicians (where every increase in PEEP was to be preceded by a recruitment manoeuvre). Physicians adjusted the inspiratory pressure to keep an adequate TV with the goal of maintaining plateau pressure (Pplat) ≤ 30 cm H2O. A change to the prone position was triggered by a finding of a PaO2/FiO2 ≤ 200 with a PEEP > 5 cm H2O or a PaO2/FiO2 ≤ 300 with a PEEP > 10 cm H2O, with FiO2 ≥ 0.6. All the patients in prone position received deep sedation and analgesia (for RASS score –5) and neuromuscular block by intravenous infusion of cisatracurium.
Data collected from medical records included the following: demographics (age, weight, body mass index [BMI]), comorbidities, ventilatory parameters (TV, respiratory rate [RR], PEEP, Pplat, driving pressure, respiratory system compliance [Crs], FiO2, ventilatory ratio, extracorporeal membrane oxygenation [ECMO]), arterial gases, Murray´s acute lung injury and Sequential Organ Failure Assessment (SOFA) scores, disease chronology (days from onset of symptoms and from hospital admission to intubation, length of stay in ICU), and complications related to the prone position.
Statistical analysis
We used descriptive statistics to summarize the clinical data. The results were reported as medians and interquartile ranges (IQR). Categorical variables were reported as counts and percentages. We compared variables across groups using Student’s t test or the Mann-Whitney test as appropriate. We reported all available data without imputation. Tests were two-sided, and a P-value < 0.05 was considered statistically significant. We performed analyses with Medcalc© software Version 18.9.1 (Medcalc software, Ostend, Belgium).
RESULTS
Clinical and demographic characteristics
From 10 March to 30 April, 2020, 94 patients with confirmed COVID-19 infection and severe respiratory failure that required endotracheal intubation were admitted to 2 ICUs of the Salamanca University Hospital. Of these patients, 84 required the placement in the prone position due to refractory hypoxaemia. The demographic and clinical characteristics of these patients, the therapies used, and the outcomes are shown in Table 1. The mean age of the patients was 67 years, and the majority (72%) were male; the most frequently associated comorbidities were hypertension (47%), obesity (31%), and diabetes mellitus (23%). Eight per cent of the patients had previous respiratory diseases and 6% were active smokers.
TABLE 1
Patient characteristics on hospital presentation
Respiratory characteristics of the patients upon admission to the ICU
The respiratory characteristics of the patients upon admission to the ICU are summarized in Table 2. At the time of admission to ICU, all patients met the Berlin criteria for ARDS, being mild in 26.2% of cases, moderate in 61.9%, and severe in 11.9%. Crs was similar between patients with a predominantly ground glass radiological pattern (34 [IQR 27–42]) and those with a predominance of condensation (33 [IQR 25–40]) (P = 0.41). After intubation, the Crs was 34 (IQR 25–43) in patients who were discharged from the ICU and 33 (IQR 25–40) in those who died (P = 0.30); at 72 h, the Crs was 34 (IQR 26–40) and 33 (IQR 27–44), respectively (P = 0.97) (Table 3).
TABLE 2
Respiratory characteristics of patients
TABLE 3
Respiratory variables after intubation and 72 h after the first prone ventilation between those alive and those who died
Response to ventilation in the prone position
The median number of cycles in the prone position was 2 (IQR 1–3), with a median of 24 hours per session (IQR 24–30) and a median of 76 total hours in the prone position (IQR 64–111).
Before prone positioning, the median PaO2/FiO2 was 105 (IQR 76–138), the PaCO2 was 49 mm Hg (IQR 42–57), the Pplat was 27 cm H2O (IQR 25–30), the driving pressure was 14 cm H2O (IQR 11–18), the PEEP was 12 cm H2O (IQR 12–14), and the Crs was 33 mL cm H2O-1 (IQR 25–41) (Figure 1). During the prone positioning, those values were, respectively, 182 (IQR 144–241), 48 mm Hg (IQR 42–55), 27 cm H2O (IQR 24–29), 14 cm H2O (IQR 11–17), 12 cm H2O (IQR 12–14), and 31 mL cm H2O-1 (IQR 25–41). After the patients were placed in the supine position, the median PaO2/FiO2 was 151 (IQR 115–201), the PaCO2 was 50 mm Hg (IQR 40–63), the Pplat was 26 cm H2O (IQR 24–28), the driving pressure was 13 cm H2O (IQR 11–16), the PEEP was 12 cm H2O (IQR 10–14), and the Crs was 33 mL cm H2O-1 (IQR 25–48). Seventy-two hours after initial prone ventilation, the median PaO2/FiO2 was 193 (IQR 152–251), the PaCO2 was 49 mm Hg (IQR 43–54), the Pplat was 26 cm H2O (IQR 23–28), the driving pressure was 14 cm H2O (IQR 11–16), the PEEP was 12 cm H2O (IQR 10–14), and the Crs was 34 mL cm H2O-1 (IQR 26–43). The percentage of patients with normal Crs (> 50 mL cm H2O-1) increased progressively with the prone position from 15% (n = 13) to 32% (n = 27) at 72 h after the first prone manoeuvre.
FIGURE 1
Respiratory indices after intubation (BASAL), after 6 h in the prone position (PRONE), 6 h after the change from prone to supine (TIME6H), and 72 h after the first cycle of prone position (TIME72H). Crs – compliance. The solid line indicates the median value
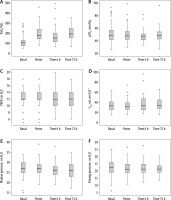
No differences were found at baseline or at 72 hours after the first prone manoeuvre in gasometry parameters or in respiratory mechanics between those patients who died and the survivors, except in the PEEP at 72 h (Table 3).
Prone position and adverse effects
Pressure ulcers were the most frequent adverse events (n = 11, 13%). Seven patients (8.3%) required a change of position from prone to supine due to endotracheal tube obstruction or poor tolerance to the prone position. Two patients (2.4%) presented an accidental loss of central venous access during position change. Corneal ulcers occurred in 2 patients (2.4%). One patient (1.2%) had a brachial plexus injury.
Outcomes
Nineteen patients (22%) were successfully extubated, and the median duration of mechanical ventilation was 11 (IQR 8–16) days. Twelve patients (14%) required reintubation. Nineteen patients (22%) had a pneumothorax requiring chest drainage, and 14 (16%) presented bacterial pneumonia. Forty-three patients (51.2%) were discharged from the ICU. Forty-one patients (48.8%) died. The length of ICU and hospital stay of the survivors was 15 (IQ 9–25) and 36 (IQR 23–47) days, respectively.
DISCUSSION
In this retrospective study we characterized 84 COVID-19 patients with ARDS managed with mechanical ventilation and prone position. Upon initiation of mechanical ventilation, the patients in our cohort had a PaO2/FIO2 of 105, a ventilatory ratio of 1.48, and a predominantly moderate degree of distress according to the Berlin criteria. The values of Crs, Pplat, and driving pressure were not different to previously published cohorts of ARDS patients with or without COVID-19 infection [3, 4, 11–13]; in addition, 85% had low Crs.
The first reports of patients with COVID-19 pneumonia and acute respiratory failure admitted to an ICU showed that COVID-19 infection does not lead to a “typical” ARDS [1]. Based on these findings, Gattinnoni et al. [2] found great variability in the Crs of these patients, which led them to postulate that there are 2 predominant phenotypes: type L, characterized by low elastance (i.e., high compliance), low ventilation-to-perfusion ratio, and low recruitability; and type H, characterized by high elastance, high right-to-left shunt, and high recruitability. Although the results of our cohort show that there is some heterogeneity, most of the patients (85%) presented ARDS with typical characteristics (low Crs), similar to those described in other studies [3, 4, 11].
Prone ventilation improves gas exchange in ARDS by several mechanisms [8]. The improvement in oxygenation with prone positioning in our cohort was consistent with prior studies of prone ventilation in early ARDS [10]. Although in our study the median of the Crs was similar at baseline and at 72 h after the prone position, the percentage of patients with normal Crs (> 50 mL cm H20-1) doubled (from 15% to 32%). Mortality in our patients was 48.8%, which is similar to that described in other series (16–97%) [4, 14, 15].
The incidence of pneumothorax requiring chest drainage was 22%, a higher figure than that reported in some studies in ARDS patients treated with mechanical ventilation in the prone position (0–9.2%) [9, 16, 17]; however, it is within the range described during mechanical ventilation of patients with non-COVID-19 ARDS (14–87%) [18]. Moreover, some authors have suggested that the incidence of pneumothorax in COVID-19 ARDS could be higher than in other causes of ARDS [19]. Several factors may have contributed to this high incidence of pneumothorax: 1) although we used a protective ventilation strategy, high PEEP levels may have played a role; 2) heterogeneous overdistention of the alveoli due to mucus impaction and/or diffuse alveolar injury caused by SARS-CoV-2 can lead to alveolar rupture, which in turn can produce air leakage; and 3) cyst formation has been described by studies demonstrating radiological progression from areas of consolidation to bullae (cyst formation has been described in patients undergoing or not undergoing positive-pressure ventilation, suggesting that barotrauma alone cannot account for these findings) [20].
Forty-seven per cent of our patients required a tracheostomy. This figure is quite close to that previously described by other authors in patients with ARDS due to COVID-19 [21]. Currently there is no evidence on when to perform a tracheostomy in those patients. Some authors suggest waiting 3 weeks (to have a better perspective of the evolution and to avoid spreading the virus to healthcare workers) while others suggests that early tracheostomy (10 days or before) in carefully selected patients with COVID-19 may optimize ICU resources in a system that is experiencing an escalating number of critically ill patients [22, 23]. Moreover, clinicians in Brazil observed that in patients with COVID-19 and severe comorbidities, earlier tracheostomy (day 4–5 of intubation) could improve prognosis [24]. In our hospital, the criteria for performing a tracheostomy are: 1) usually not before 10 days, and 2) when no more prone manoeuvres are expected (except if the patient presents very thick secretions with continuous obstruction of the endotracheal tube). The average time from intubation to tracheostomy in our ICUs was 14 days (similar to or longer than the times published in other series) [23, 25]. It is also possible that the collapse of our ICUs that occurred in the first COVID-19 wave in Spain (57 patients intubated simultaneously with only 32 ICU beds) motivated us to be more aggressive in performing tracheostomy earlier in some patients, in an attempt to reduce the care pressure by facilitating the weaning and discharge of patients.
Our work has several limitations. This is a retrospective study with few patients, and it was carried out in a single hospital. Second, the ventilatory parameter settings may have varied among the different physicians due to the lack of use of a formal PEEP titration table. For these reasons, larger studies would be needed to confirm these findings.




