In Poland, over 2.5 million surgical procedures are carried out annually, 1.5 million of which are performed under general anaesthesia [1]. Thanks to the advances in anaesthesiology, the highest-risk patients can be safely anaesthetised, provided that the therapeutic options are adjusted to the patient`s individual needs. Goal-directed therapy (GDT), based on complex haemodynamic monitoring, is more beneficial for patients, mainly by reducing the risk of complications [2]. GDT aims at providing an oxygen carrier and adequate regional flow, thus optimising tissue perfusion [3].
Comprehensive interpretation of all the haemodynamic monitoring data combined with the clinical picture enables early identification of the developing disorders, determination of their pathomechanisms and selection of the management option based on the verified protocols. The consensus statement of the Cardiothoracic Anaesthesia Section of the Polish Society of Anaesthesiology and Intensive Therapy (PTAiIT in Polish) published in 2017 regarding optimisation of cardiovascular function in the perioperative period in patients undergoing non-cardiac surgery [4, 5] defined the principles of selecting the method of haemodynamic monitoring based on the individual patient risk and the procedure-related risk; moreover, the therapeutic management algorithm was suggested.
The aim of the present study was to evaluate clinical practice in intraoperative haemodynamic monitoring in selected Polish hospitals in relation to the estimated individual risk as well as the mode and type of procedure.
METHODS
A point prevalence study (PPS) was carried out under the auspices of the Intensive Therapy Section of PTAiIT. Considering the non-interventional study design, the Bioethics Committee did not require informed consent for participation in the project (KNW/0022/KB/212/19). The project was registered in the Research Registry (UIN researchregistry5176).
Selection of patients
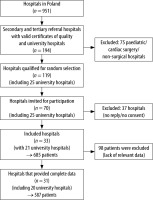
The study centres were recruited from Polish specialist secondary and tertiary referral hospitals accredited by the Centre for Quality Monitoring in Health Care (February 1st, 2018) and university hospitals (n = 194). The exclusion criteria included paediatric and cardiac surgery centres (due to different perioperative management protocols) and non-surgical centres (n = 75). From the created list (n = 119), 45 non-university hospitals were selected (simple random selection) and 25 university hospitals were added; in the period between 01.02.2018 and 01.04.2018, written invitations to participate in the study were sent two twice to the selected hospitals. The positive response was obtained from 33 centres, including 21 university hospitals. The participation rate was 27% (12/45) for non-university hospitals and 84% (21/25) for university hospitals.
In the hospitals included in the study, local coordinators were appointed; the project authors (AJS, ŁJK) acquainted them with the research procedures and project documentation. It was assumed to obtain data from patients undergoing general and/or regional anaesthesia for scheduled and emergency procedures performed during off-duty hours (according to the American Society of Anesthesiologists, category “E” ASA PS). The study did not include patients undergoing analgosedation and provided with monitored anaesthetic care.
Data acquisition
The study protocol included the basic demographic data (gender, age, body weight and height) and the clinical data regarding the preoperative risk, type of surgery, its duration, type of anaesthesia, type and dose of intraoperative fluids (balanced crystalloids, 5% and 10% glucose, 0.9% sodium chloride solution, synthetic colloids, (gelatine and carboxyethylated starch), natural colloids (blood and blood-derived preparations), type and dose of vasoactive and ionotropic drugs, method of haemodynamic monitoring, baseline electrocardiographic recording, values of static parameters of the cardiovascular function, including systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR) – baseline (the first measurement in the operating theatre once monitoring has been initiated), before and after the induction of anaesthesia and at 30-minute intervals until the patient is discharged from the operating theatre. According to the protocol, prior to discharge the patient conditions should be assessed using a modified Aldret scale.
The study was scheduled for April 5, 2018. During two weeks after finishing the study, 685 completed questionnaires were received from 33 hospitals. After verifying the completeness of data, 587 protocols from 31 hospitals (including 20 university hospital) were analysed. The process of recruitment was presented in Figure 1.
Risk assessment
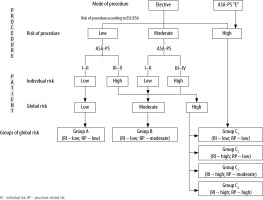
Based on the obtained data, a retrospective stratification of preoperative risk was performed. The individual risk of patients was assessed based on ASA-PS score (according to the statement of Cardiothoracic Anaesthesia Section of PTAiIT). ASA-PS III-V patients constituted a high individual risk group. Surgical procedures were classified as low, moderate or high risk, according to the guidelines of the European Society of Cardiology/ European Society of Anaesthesiology (ESA/ESC) [7]. Emergency procedures (ASA-PS “Emergency” category) were classified as high-risk procedures.
Based on individual and surgery-related risk, global risk and global risk groups (including the components of global risk) were determined (Figure 2)[4].
Statistical analysis
Statistical analysis was performed using licenced MedCalc v.18.2.1 software (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2018). Quantitative variables were presented as median and interquartile range (IQR). Qualitative variables were presented as absolute values and percentage. Inter-group differences for quantitative variables were analysed using non-parametric tests (U Mann-Whitney or Kruskal-Wallis). The χ2 test or Fisher’s test was applied for qualitative variables. P < 0.05 was considered statistically significant.
RESULTS
The study group included 587 patients (315 women, 272 men). The median age was 58 years (IQR 40–67); the number of high-risk patients was 141 (24%). The high procedure type risk regarded 17 (3%) individuals while 62 (11%) were characterised by high procedure mode risk. High global risk was observed in 186 (32%) patients. The detailed characteristics of the study group were shown in Table 1.
TABLE 1
Data on patients and surgical procedures, including risk assessment
Quantitative variables were presented as median and interquartile range (IQR). Qualitative variables are presented in absolute values and percentage.
Basic haemodynamic monitoring, including non-invasive blood pressure (NIBP) was performed in 562 (96%) patients. Non-basic methods (i.e. extended or advanced) were used in 25 patients (4%). Invasive blood pressure (invasive blood pressure – IBP) was implemented in 19 patients (3%), mostly during procedures lasting over 120 minutes (n = 16). Central venous pressure (CVP) was measured in 6 patients (1%) and pulse contour analysis in 2 patients (0.3%). Moreover, uncalibrated dilution methods, transpulmonary thermodilution, ultrasound (transthoracic and transoesophageal Doppler) and Swan-Ganz catheters were not applied. Lactate concentration (Lac) and base deficiency (BD)/excess (BE) were determined in 11 (1.9%) and 2 (0.3%) patients, respectively. The dioxide gap (pCO2 gap) and venous/mixed blood saturation, i.e. S(c)vO2 were not analysed.
The analysis of individual monitoring methods in relation to the preoperative risk was presented in Table 2. Monitoring in accordance with the Cardiothoracic Anaesthesia Section of PTAiIT [4, 5] was used in group A (low risk) and group B (moderate risk) and 10% of patients of group C (high risk).
TABLE 2
Risk monitoring methods
Quantitative variables – presented as median and interquartile range (IQR). Qualitative variables – presented as absolute values and percentage
Patients monitored intraoperatively with more advanced methods received significantly lower fluid amounts (P < 0.001) (Table 3).
TABLE 3
Intraoperative fluid therapy according to haemodynamic monitoring
In clinical hospitals (n = 21) more advanced moni-toring compared to NIBP was used more often in non-clinical centres (n = 4), most often during vascular surgery (n = 5) and abdominal surgery (n = 5).
Perioperative blood pressure and heart rate values are shown in Table 4.
TABLE 4
Perioperative blood pressure and heart rate
The percentage of patients with MAP values deviated by > 20% from baseline (at least one measuring point) was 68%. A decrease and/or increase in MAP > 20% from baseline was observed in 65% and 4% of patients, respectively; in 221 patients (40%) a decrease in MAP > 20% below baseline was observed (Table 5). A decrease in MAP < 65 mm Hg in the period from induction of anaesthesia completion of surgery was found in 122 (21%) patients, most often in those undergoing general anaesthesia (96 cases) and in high global risk procedures (52 cases).
TABLE 5
Percentage of individual mean arterial pressure values deviating from baseline by > 20% before induction of anaesthesia
Among vasoactive and inotropic drugs, ephedrine was most commonly used; it was administered to 143 patients (24%), 15 mg (IQR 10–25) i.e. 0.18 mg kg-1 (IQR 0.11–0.3). It was most frequently given to patients undergoing high-risk global procedures (n = 63) that lasted < 120 minutes (n = 110 vs. n = 33), patients hospitalized in clinical centres (n = 93 vs. n = 50) and provided with intraoperative basic monitoring (n = 130 vs. n = 13). The use of ephedrine was different in procedures under general (20% of patients, n = 79), regional (31%, n = 54) and when both types of anaesthesia were combined (56%, n = 10) (P < 0.001). In the study group, noradrenaline (n = 12), adrenaline (n = 1) and isoprenaline (n = 1) were rarely used. Dopamine, dobutamine and milrinone were not used. Intraoperatively, patients were only occasionally administered atropine (n = 13), urapidil (n = 6) and metoprolol (n = 2). Nitroglycerin was not used.
DISCUSSION
Our point prevalence study was the first multi-centre study in Poland to assess clinical practice in intraoperative hemodynamic monitoring. NIBP was found to be most frequently used; other tools were used sporadically. Although 32% of patients were at high risk of complications, only in 10% of them, intraoperative decisions were based on more advanced protocols. From amongst 24% of patients receiving intraoperative ephedrine, only 9% of patients were monitored using tools other than NIBP. In patients with basic monitoring the fluid intake was significantly higher.
According to the euSOS study, in Europe 1 in 10 patients undergoing major abdominal surgery is monitored for cardiac output [8]. Several surveys analysed the preferences of anaesthesiologists regarding methods of monitoring in high-risk patients [9–12]. A questionnaire study involving anaesthesiologists from Europe and North America (n = 368) has demonstrated that 34% of physicians monitor cardiac output during anaesthesia, and more than 80% use CVP measurements [9]. Polish anaesthesiologists are less likely to use extended methods of monitoring in high-risk patients, as compared anaesthesiologists from other European countries or the United States. Likewise, monitoring of cardiac output in the study centres was incidental, constituting up to 1.5% of patients at high perioperative risk. Only 17% of high-risk patients were monitored for IBP, as compared to 89% from other parts of Europe and 95% from the United States. Moreover, Polish anaesthesiologists in the study centres monitored CVP equally rarely (9% vs. 72%). A similar study in Japan (n = 573) has shown that only 20% of anaesthesiologists consider an increase in cardiac output as an indicator of positive response to fluid therapy, while 80% take fluid decisions based on BP and hourly diuresis. As much as 98% of Japanese anaesthesiologists declare that they monitor IBP in high-risk patients [10]. Similar data were provided by a survey conducted in China (n = 210) [11]. Our study was based on the analysis of the procedures performed, and not only on doctors’ preferences, therefore, it provides real insight into anaesthesiologic practice.
The maintenance of hemodynamic stability during anaesthesia significantly reduces the incidence of complications and death [13]. While the upper value of SBP (considered to be relatively safe) varies between reports [14–16], the growing amount of data indicates that even a short-term decrease in SBP < 100 mm Hg and MAP < 60–70 mm Hg may be harmful to patients undergoing noncardiac surgeries [16–19]. The protocol of our study assumed BP measurements at 30-minute intervals; nevertheless, even at that long intervals intraoperative hypotension, defined as a decrease in MAP by > 20% from baseline, was observed in 65% of patients. Due to the type of our study i.e. PPS, it is, however, difficult to link this fact with therapeutic decisions.
Hemodynamic stability is essential in high-risk patients with limited physiological reserves [20]. In our study, only in 0.3% of patients cardiac output was monitored, and in 2% of patients indirect microcirculation markers were determined – a disproportion between the PTAiIT statement [4, 5] and clinical practice in the high-risk group was significant. The results of large meta-analysis reveal that the use of GDT based on reliable haemodynamic monitoring reduces the incidence of postoperative complications and mortality in high-risk patients [21–23]. Further studies are required to determine the GDT effects on the reduction of mortality in lower-risk groups [24]. A meta-analysis of Spanish researchers shows that GDT based on transoesophageal echocardiography (Doppler) was associated with fewer perioperative complications in high-risk patients, yet similar effects were achieved with restrictive fluid therapy. Such benefits have not been demonstrated in patients at moderate risk [25]. In order to meet numerous doubts related to the use of rigid GDT protocols, Saugel and Vincent have suggested a Protocolised Personalized Peri-operative Haemodynamic Management (PPPHM) strategy aimed at establishing strictly individualized perioperative procedures [26]. The need for functional hemodynamic monitoring and titration of hemodynamic effects depending on the cardiovascular pathophysiology of individual patients was highlighted, which is infeasible when the condition of a high-risk patient is monitored solely based on non-invasive blood pressure measurements.
The point prevalence, cross-sectional studies clearly demonstrate daily practices yet have some limitations. Firstly, when assessing the perioperative strategy, the differences in management in specific surgical procedures should be considered. Secondly, our study did not include the analysis of contraindications for the use of selected monitoring methods, which, however, cannot justify the rare use of widely available and relatively safe methods, such as IBP. Thirdly, we cannot exclude the situations in which the anaesthetic team abandoned a given method of invasive monitoring as its implementation was associated with an inappropriate risk-benefit ratio or a delay of the life-saving procedure. Fourthly, the study was conducted within one day during off-duty hours (i.e. the basic hospital ordinance), according to its methodology. It is likely that a several-day or a week study involving 24 hours/day would provide different results. In our project there was a visible overinterpretation of university hospitals with a much lower participation of non-university hospitals, which could also affect the results. Fifthly, observational studies do not allow to assess a causal relationship; therefore, it is difficult to link the nature of the observed hemodynamic changes (mainly hypotension) with the therapeutic interventions undertaken. Finally, our findings can hardly be compared with European practice. Therefore, the conclusions about discrepancies between recommendations regarding hemodynamic monitoring and clinical practice should be formulated with extreme caution. We can only suppose that this situation results from inadequate financing (valuation) of anaesthetic services by the national payer. Moreover, the lack of knowledge of current recommendations or the characteristics of the labour market in our country may also play a certain role -anaesthesiologists employed on a contract basis rotate quite dynamically and the implementation of medical procedure standards requires time, continuous training and monitoring of its effects.