According to currently available data, from 5% to 16% of patients tested positively for COVID-19 required admission to an intensive care unit (ICU) due to respiratory failure and the necessity to introduce mechanical ventilation [1, 2]. Respiratory failure is the dominating medical issue in the course of this disease. It is widely acknowledged that computed tomography is the gold standard for diagnosing lung diseases, and it facilitates the visualisation of interstitial inflammatory lesions that are found also in COVID-19 [3]. In such cases chest X-ray is characterised by a significantly lower sensitivity and speci-ficity. However, in numerous reports from China, Italy, and the USA physicians emphasise the efficacy of ultrasound both during the preliminary diagnostic process and then when monitoring disease dynamics in COVID-19 patients [4–6].
Lung ultrasound has been utilised in the framework of point-of-care (POC) for years and is a reco-gnised diagnostic tool in this context [7]. An ultra-sound examination performed by a clinician perfectly complements data obtained during the physical examination and plays a similar role to that of chest auscultation. An ultrasound transducer replaces the stethoscope and becomes a “sonoscope”, and the number of valuable data that can be obtained with this modality is much larger as than for chest auscultation. POC lung examination is one of the least complicated ultrasound applications, and, as shown in multiple studies, theoretical and practical training lasting a few hours is sufficient to attain basic skills in performing this examination [8].
The present pandemic and the risk associated with spreading the infection have revealed one more important advantage of ultrasound: the possibility of performing a comprehensive bedside examination with the use of a portable device. Traditional physical examination is limited by personal protective equipment (PPE), and the transportation of the patient to an imaging diagnostic department is a serious logistical challenge that engages already limited human resources and creates the risk of transferring the infection outside the ICU. The issues related to the epidemiological safety and the rationing of PPE necessitated by its limited availability additionally prompt the reduction of medical staff entering the red zone to the minimum numbers necessary.
Widespread accessibility to portable ultrasound devices in the ICUs provides the attending physician with the means for a quick bedside diagnosis of the reasons for a rapid deterioration of the patient’s condition (e.g. caused by the development of pneumothorax) and the introduction of causal treatment [7].
Currently, for obvious reasons, the number of publications devoted to the utilisation of lung ultrasound in COVID-19 patients is scarce. Provisional guidelines are based on reports offered by recognised intensivists during webinars organised by prestigious medical societies [9]. However, the published guidelines are often inconsistent and internally contradictory, which leads to confusion. The aim of this Consensus is to discuss a broad spectrum of aspects associated with the utilisation of lung ultrasound in the ICUs for COVID-19 patients.
REQUIRED QUALIFICATIONS
Patient safety should be of the utmost importance; hence, staff using ultrasound as a diagnostic tool should be adequately trained.
Presently, no specific legal regulations apply in Poland with respect to the performance of ultrasound examinations, including point-of-care lung ultrasound (POC-LUS).
Consistent with the legal opinion of 20 July 2017 commissioned by the Polish Ultrasound Society, the situation is as follows: In the current legislation, there are no provisions that would require physicians, including those offering commercial medical services (contract physicians), to possess any specific qualifications that would authorise them to perform ultrasound examinations. No legal regulation enumerates medical specialties the completion of which would univocally grant the right to perform this type of diagnostic examination. No legally established catalogue of certificates that would grant such qualifications to physicians now exists.
Consequently, physicians are obliged to obey the rules of professional conduct as provisioned by the Act of 5 December 1996 on the professions of doctors and dentists1 complemented by Article 10, item 1 of the Medical Code of Ethics2, stipulating as follows: “Physicians shall not go beyond their professional competence and skills while performing diagnostic, preventive, treatment, and certifying activities”.
What does this mean in the context of the SARS-CoV-2 pandemic? This means that there are no legal restrictions as regards the utilisation of lung ultrasound in preliminary diagnosis and monitoring of COVID-19 patients. We should follow the already available experience of physicians from China or Italy, who unequivocally stress the importance of transthoracic lung examination and the broadly understood significance of point-of-care ultrasound, i.e. a more holistic use of ultrasound, for intensive care management of patients in ICUs. We should take advantage of this know-how under our existing circumstances.
Is this a good time to learn lung ultrasound exa-mining techniques and to utilise the potential of lung ultrasound in preliminary diagnosis and monitoring of COVID-19 patients? We will have no better opportunity for a large number of physicians to become familiar with this method of lung imaging and apply it in their everyday clinical practice. It should be stressed that presently many anaesthesiologists and intensivists use this method already, and – what is most important – this is in compliance with Polish legislation.
EQUIPMENT REQUIREMENTS
Ultrasound device
To perform lung ultrasound any ultrasound device that offers B-mode imaging is sufficient. The availability of other imaging modes (M-mode, colour/spectral Doppler) is not necessary. Battery-powered portable machines make it possible to switch on, prepare, and set the device in the clean zone and then enter the patient’s zone only for the time necessary to perform the examination. In such conditions portable and ultramobile devices may be particularly useful. Images/clips should be recorded during the examination on the hard drive of the device or transferred wirelessly to the computer to enable data analysis outside the examination zone and their digital archiving. For the duration of the examination transducers and cables that are not used should be detached and all unnecessary accessories removed. A device with a touch panel or a small number of buttons/knobs is much easier to effectively clean and disinfect after the completed examination.
Choice of the transducer
The following transducers are used for lung ultra-sound examination:
Convex/micro-convex. It facilitates a comprehensive examination of the lungs, including: the assessment of artefacts, pleural line, and subpleural lesions of supradiaphragmatic regions, and pleural cavities, due to the deep penetration of the ultrasound beam and good near-field resolution.
Linear. It offers a high-resolution image with a penetration limited to 4–5 cm. It is particularly useful for the assessment of the pleural line and the subpleural region, and the verification of lung sliding. In adult patients the assessment of supradiaphragmatic abnormalities and pleural cavities may be difficult/impossible. In paediatric patients a comprehensive lung examination is possible with a linear transducer.
Phased array. It offers a deep range of penetration. Due to its construction, it provides an image characterised by a narrow near field, which makes it difficult/impossible to assess in detail the pleural line and subpleural lesions. It facilitates the good visualisation of deep structures and assessment of artefacts.
Optimisation of the device/transducer settings
In order to obtain a diagnostic ultrasound image of the lungs it is recommended that the following steps are taken:
switch off filters that make artefacts more difficult to distinguish (tissue harmonic imaging [THI], compound imaging), filters for image smoothing and speckle reduction, or similar ones. This increases the clarity of artefacts,
reduce the gain in order to improve artefact visi-bility,
set the depth range as follows: adult patients – 10–15 cm, paediatric patients – 5–10 cm,
set the frequency range: general for a particular transducer,
choose a single focal zone and set it at the level of the pleural line.
Predefined transducer settings optimised for the assessment of the lungs (the so-called lung presets) are recommended. The use of presets shortens the device preparation time and ensures comparable examination conditions. If a preconfigured lung preset is unavailable, in order to assess the lungs the abdominal preset should be modified according to the aforementioned guidelines [10–15].
STAFF AND TRANSDUCER PROTECTION, ULTRASOUND DEVICE CLEANING
The use of appropriate PPE is a key element of the care provided for patients with suspected or confirmed diagnosis of COVID-19 treated in an ICU. Because an ultrasound examination requires direct contact between clinicians and patients, it is necessary to use full PPE:
Patients who are not intubated should wear a medical mask (if tolerated) during the examination.
During the examination of patients suspected/diagnosed with COVID-19, the following rules should be obeyed in order to reduce contamination risk:
the examination protocol described below should be followed; images and cine-loops should be recorded for further analysis,
single-use ultrasound gel dedicated for one patient should be applied,
PPE should be worn consistently with the above list,
disposable covers and sleeves for the ultrasound device and transducers should be used,
after the examination and preliminary disinfection of the covers with alcohol disinfectant, they should be removed from the ultrasound device and transducers. Next, a comprehensive disinfection of the entire device (including the wheels) and transducers should be performed,
an ultrasound device dedicated only to COVID-19 patients (if possible) is recommended.
LUNG ULTRASOUND PROTOCOL FOR PATIENTS SUSPECTED OR DIAGNOSED WITH COVID-19
Examination in the ICUs is usually performed on patients in the supine position. Moreover, the body area available for examination may be limited due to the inserted cannulas, dressings, drains, and parts of the monitoring equipment. The examination should cover the largest possible area of the lungs accessible.
The examination begins with the convex transducer to assess lesions affecting the large respiratory area of the lungs, e.g.: B-line artefacts occurring over a large area, lobar consolidations, and a large volume of fluid in the pleural cavity. The chest surface available for examination is scanned, moving the transducer placed along the intercostal space, from the apex of the lungs to their base, in consecutive body lines: parasternal, midclavicular, axillary (anterior, midaxillary, and posterior) (Figure 1). The assessment of the lateral-posterior region requires a precisely performed examination because the majority of gravitational abnormalities are found there. We stress that the suggested technique recommends the placement of the transducer along the intercostal space and not a longitudinal placement in relation to the body axis, as used normally. The technique is modified due to the necessity to adapt it to the circumstances under which the exa-mination is performed in the ICU and the patient’s status. When interstitial lesions are visualised, the examination should be repeated with a linear transducer. This will facilitate a detailed evaluation of the abnormalities within the pleural line.
The duration of the examination depends on the clinical needs and the number of posed questions. For COVID-19 patients, lung examination should take as long as necessary to obtain diagnostic ultrasound images. In order to do so, it should be performed consistently with the described protocol, and video cine-loops should be recorded. Then, after leaving the patient’s zone, the recordings can be analysed again. Another advantage of recording the video material is the possibility to consult the ultrasound images with a more experienced member of the medical team, as well as to return to the initial material to compare and assess it during the patient’s course of monitoring.
The visualisation of abnormalities in lung ultrasound also allows for the monitoring of patients requiring extracorporeal membrane oxygenation (ECMO) due to acute respiratory distress syndrome (ARDS). Mongodi et al. [16] proposed a protocol for the assessment of the type and range of particular ultrasound pathological findings, including a score assessing the severity of pulmonary lesions.
EXAMINATION DESCRIPTION AND DOCUMENTATION
POC-LUS performed within the framework of observation and diagnostic activities should be duly documented. POC examination is regarded as one element of the comprehensive examination, having an equal status to auscultation with a stethoscope, transcutaneous saturation, ECG monitoring, etc. The documentation should include a description of the performed examination and its result as part of the observation information content. The examination description should include the following: date, hour (due to the possibility of performing the examination repeatedly during a 24-hour period), and types of transducers used. Moreover, before the description of the examination result, comments as to the factors that might have limited the imaging itself should be provided, e.g.: the patient’s position, obesity, dressings. The second part of the description should include data concerning ultrasound images and conclusions. Lung assessment performed as a POC examination should account for the following:
lung sliding sign,
pathological artefacts and their localisation,
subpleural consolidations and their localisation,
fluid in pleural cavities.
When describing the examination, video recorded cine-loops are extremely helpful. During the patient’s monitoring they are particularly useful and allow for the assessment of lesion evolution.
NORMAL LUNG IMAGE
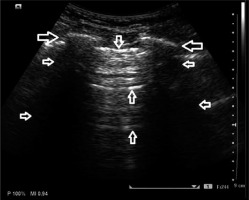
Sonographic features of normally aerated lungs include the presence of lung sliding and A-line artefacts [17].
Lung sliding results from the normal movement of the pleural layers and represents a layer of visceral pleura sliding on parietal pleura. It is visualised as a lateral movement of the pleural line consistent with the patient’s respiration. Normally, the pleural line is a thin continuous hyperechoic line. Its thickness does not exceed 2 mm, and the measurement is taken with a linear transducer. In order to unmistakably identify the pleural line, the bat sign is helpful. To this end, the transducer should be placed over the chest wall along the longitudinal body axis to visualise two consecutive ribs and acoustic shadowing behind them. The pleural line is located between the ribs and about 3–5 mm below the rib line.
A-line artefacts are reverberation artefacts, and they indicate normally aerated lungs (Figure 2). They are formed as a result of the repetitive reflection of the ultrasound beam by the pleural line. They are visualised as hyperechoic horizontal lines below and parallel to the pleural line, always at the same distance (equal to the distance between the body surface and the pleural line).
ABNORMALITIES IN THE COURSE OF COVID-19 IN INTENSIVE CARE UNIT PATIENTS
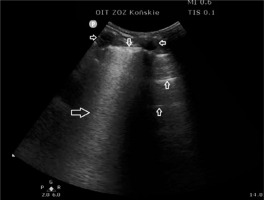
Characteristic abnormalities that may occur in the SARS-CoV-19 infection include the following: B-line artefacts of various intensity, abnormalities within the pleural line, and the presence of subpleural consolidations [18] (Figure 3–5).
FIGURE 3
Interstitial lung lesions in lung ultrasound; (↓) pleural line, (←) vertical artefacts – so-called B-lines
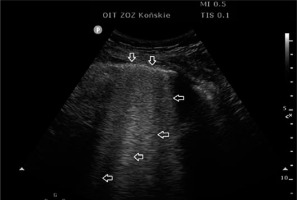
FIGURE 5
Sub-pulmonary consolidations with a diameter of about 5 mm (↓), accompanied by vertical artefacts coming from the lower edge of the sub-pleural changes; so-called C-line artefacts (→ ←)
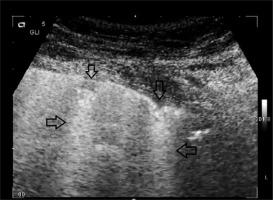
B-line artefacts are hyperechoic vertical artefacts arising from the pleural line, extending to the bottom of the screen (irrespective of the set depth), and moving along with the movements of the pleural line Figure 3. Consistent with available reports, pathology is identified when more than two B-lines are found in one intercostal space (in a longitudinal scan), but this refers to the diagnosis of cardiogenic pulmonary oedema. Thus far literature reports have not discussed the norms relating to the number of B-line artefacts in various age groups and in clinical conditions other than pulmonary oedema. In the initial phase of interstitial pneumonia, the number of focal B-lines is unpredictable. Consequently, any number of untypically localised B-lines should be regarded in the current state of pandemic as a pathological ultrasound finding. In the course of COVID-19, both single and multiple B-line artefacts may appear, visible over the entire lung surface [19]. The more numerous they are, the more intensified the pathological process, and areas of properly aerated lung (so-called “spared areas”) are focal (Figure 4). Their presence indicates the pathological process affecting the interstitium. Such findings may occur in interstitial pneumonia induced by viral infections. As the pathologies intensify, B-line artefacts merge and a broad hyperechoic band is formed – the “white lung” sign. Abnormalities within the pleural line most often involve its irregularity and discontinuity, which may be associated with the presence of tiny subpleural lesions (Figure 5). Consolidations are airless areas of the lungs, mostly of reduced echogenicity, adjacent to the pleural line. In the course of COVID-19 small consolidations may develop, mostly in posterior-inferior and lateral sections of the lungs. According to available reports, pleural effusion and large lobar consolidations are very rare in COVID-19. However, they may occur in secondary bacterial infection. Lung ultrasound facilitates the monitoring of the exacerbation and alleviation of the enumerated abnormalities.
COVID-19 AND COMORBIDITIES
Pathological findings visualised in lung ultrasound in the course of COVID-19 are typical of any interstitial pneumonia; however, they are not specific to coronavirus infection. Similar abnormalities may be observed also in other viral infections or those of an atypical aetiology [20].
COVID-19 patients who require intensive care treatment are most frequently elderly patients with comorbidities. Heart failure in this patient group constitutes one of the most frequent causes of hospitalisation, amounting to 6–10% in the geriatric population and over 10% in patients older than 75 years [21, 22]. Consequently, in the case of hospitalised COVID-19 patients, it should be remembered that pathologies affecting the interstitial space may overlap. As a result, ultrasound findings will include multiple B-line artefacts, secondary to both pathological conditions. In cardiogenic pulmonary oedema, B-lines cluster gravitationally, bilaterally, usually symmetrically, and the pleural line is free of abnormalities. Compression atelectasis caused by the accumulation of fluid due to heart failure may be visualised in the parabasal region [23]. In patients who exhibit overlapping abnormalities associated with oedema due to cardiogenic and non-cardiogenic causes, the differential diagnosis based on the ultrasound findings is largely limited. Clinical data and the assessment of the heart in echocardiography are useful for the differential diagnosis, as well as the monitoring of the effects of therapy targeted at improvement of circulatory system efficiency.
Similar observations relate to patients with interstitial lung disease involving fibrosis, whose percentage in the general population is much lower than those with heart failure [24]. However, in these patients B-line artefacts are also the leading ultrasound finding. Regarding patients with interstitial lung disease involving fibrosis, it should be remembered to assess abnormalities within the pleural line that cause the emergence of B-lines and their localisation. Generally, abnormalities dominate in the lower lung fields and are less clearly expressed in the middle and upper fields, although this depends on the disease advancement [22].
In the course of viral pneumonia, secondary bacterial infection may develop. Ultrasound findings of bacterial pneumonia include large consolidations affecting a significant part of the lobe, the entire lobe, or a few lobes. Within the consolidation the so-called dynamic air bronchogram is visible, and further ultrasound findings include the following: fluid bronchogram, air trap sign, hypoechoic pleural line, and pleural effusion.
In order to efficiently monitor a COVID-19 patient with the use of ultrasound, the first examination should be performed right after the initial stabilisation of the patient. Subsequent examinations will be then referred to the initial image. The participation of the entire, adequately trained team is the key to a successful ultrasound monitoring of the disease course.
BASIC ECHOCARDIOGRAPHIC ASSESSMENT DURING ASSISTED VENTILATION IN COVID-19 PATIENTS
Bedside echocardiography is a very useful modality that may facilitate the assessment of haemodynamic parameters in patients with respiratory failure [25]. In COVID-19 patients, due to epidemiological restrictions and the critical medical condition of those patients who require life support (e.g. mechanical ventilation, extracorporeal therapies), cardiac ultrasound will differ from a formal echocardiography that is normally performed by a cardiologist. The examination performed in the ICU by the attending physician is contextual and must be interpreted while taking into account the full clinical image [26].
Echocardiography in mechanically ventilated patients due to severe pneumonia should be aimed at a dynamic evaluation of the interaction between the circulatory system and the respiratory system [27]. Such a specificity of the examination stems from the necessity to assess the effect of positive pressure in the chest cavity generated by the ventilator on the function of the patient’s circulation (i.e. impaired venous return and its negative impact on the preload and afterload of the left and right ventricle) [28, 29]. It seems that bedside echocardiography may contribute to the optimisation of the ventilation and the choice of therapy targeted at the circulatory system.
Recommendations concerning bedside echocardiography for the assessment of COVID-19 patients are as follows:
Bedside transthoracic echocardiography (TTE) should be the examination of choice due to its versatility and accessibility [30].
TTE facilitates the identification of the cause of haemodynamic instability, even by a physician who possesses only basic competence in its application [31, 32].
Basic signs that should be identified/assessed during TTE in haemodynamically unstable patients are as follows:
A regular evaluation of the circulatory system during mechanical ventilation may be performed in order to optimise ventilation parameters and to minimise the negative effect of positive pressure in the chest on the patient’s haemodynamic status [39].
When assessment with the use of TTE is impossible or only partly possible, transoesophageal echocardiography (TEE) should be considered [40, 41].
Detailed echocardiographic evaluation during mechanical ventilation should include the following:
quantitative assessment of diastolic and systolic left ventricular function [42],
quantitative assessment of systolic right ventricular function [43],
assessment of valvular function,
assessment of pulmonary artery pressure [44],
assessment of the efficiency of therapeutic interventions, i.a. fluid therapy and vasoactive drugs [45].
TRAINING
The SARS-CoV-2 pandemic and hospitalisation of many patients with symptoms of severe pneumonia secondary to COVID-19 allows physicians working in the ICUs to follow the disease dynamics with the use of ultrasound. Each performed examination is an educative experience for an intensivist because, despite lesions typical of interstitial inflammation, COVID-19 is characterised by an unknown clinical course thus far. If possible, efforts should be made to ensure that medical staff with more experience in performing POC ultrasound trains those less skilled. This will reduce the risk that the absence of physicians who perform ultrasound examinations might completely prevent any possibility of bedside imaging diagnostics.
The SARS-CoV-2 pandemic has arrived in different countries at various stages and paces. Due to the exchange of information via modern media it has been possible to forward, for instance, recorded ultrasound scans of COVID-19 patients hospitalised in China and Italy before the first patients appeared in other countries [4, 6].
Online conferences (webinars) devoted to, among others, COVID-19 in intensive care have shown that this form of education may be successfully applied in the teaching and learning of lung ultrasound techniques. This is one of the most valuable experiences gained during the pandemic, which may be of use in the future.
FURTHER PERSPECTIVES – WHAT COMES AFTER COVID?
The SARS-CoV-2 pandemic has led to a rapid increase in the interest in POC ultrasound as a versatile bedside diagnostic tool that allows clinicians to comprehensively examine critically ill patients. Currently, no consensus of national medical societies exists as concerns POC ultrasound in everyday clinical practice. Reports and first publications from countries in which POC ultrasound has an established position as a diagnostic tool and where validated training systems exist indicate that this method has been successfully introduced not only in the ICUs, but also at the triage stage in Emergency Units [6, 9].
It appears justified and actually indispensable to design and introduce shortly a similar system of education in this respect, based on already existing and tested models.