Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
PHARMACOLOGY AND PHARMACY / CLINICAL RESEARCH
Contribution to determining the antioxidant capacity of melatonin in orodispersible tablets – comparison with reference antioxidants
1
Pharmacy and Pharmaceutical Technology Department, School of Pharmacy, University of Granada, Granada, Spain
2
Zoology Department, School of Science, University of Granada, Granada, Spain
Submission date: 2017-06-05
Final revision date: 2017-10-05
Acceptance date: 2017-10-10
Online publication date: 2020-04-05
Publication date: 2020-05-26
Arch Med Sci 2020;16(4):871-877
KEYWORDS
melatoninFRAPantioxidant activityTACFDDT´sAntioxidanttotal antioxidative capacityferric ion reducing antioxidant powerfast dissolving/disintegrating tablets
TOPICS
ABSTRACT
Introduction:
Melatonin is a hormone used in the treatment of diverse pathologies due to its ability to regulate numerous physiological processes related to biological rhythms and neuroendocrine function.
Material and methods:
This study examines whether or not the antioxidant capacities of melatonin are modified during the creation of fast disintegrating oral tablets (FDDTs) through direct compression in which different concentrations of the active substance (3, 5, 10 and 60 mg) and excipients are made. In addition, the antioxidant capacity of melatonin is compared with that of reference antioxidants such as vitamin C, Trolox, resveratrol, glutathione and nicotinamide adenine dinucleotide phosphate (NADPH).
Results:
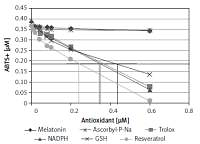
Melatonin has a lower antioxidant capacity only 5% of the capacity of resveratrol. Resveratrol is the compound having the greatest antioxidant capacity. As for the influence of the tablets components, it was found that only at higher concentrations of melatonin (60 mg/tablet), with the excipients mannitol, polyvinylpyrrolidone (crospovidone), magnesium stearate and anhydrous colloidal silica, did a decrease occur in the antioxidant capacity value, possibly due to the lower percentage of these excipients in the formulation.
Melatonin is a hormone used in the treatment of diverse pathologies due to its ability to regulate numerous physiological processes related to biological rhythms and neuroendocrine function.
Material and methods:
This study examines whether or not the antioxidant capacities of melatonin are modified during the creation of fast disintegrating oral tablets (FDDTs) through direct compression in which different concentrations of the active substance (3, 5, 10 and 60 mg) and excipients are made. In addition, the antioxidant capacity of melatonin is compared with that of reference antioxidants such as vitamin C, Trolox, resveratrol, glutathione and nicotinamide adenine dinucleotide phosphate (NADPH).
Results:
Melatonin has a lower antioxidant capacity only 5% of the capacity of resveratrol. Resveratrol is the compound having the greatest antioxidant capacity. As for the influence of the tablets components, it was found that only at higher concentrations of melatonin (60 mg/tablet), with the excipients mannitol, polyvinylpyrrolidone (crospovidone), magnesium stearate and anhydrous colloidal silica, did a decrease occur in the antioxidant capacity value, possibly due to the lower percentage of these excipients in the formulation.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.