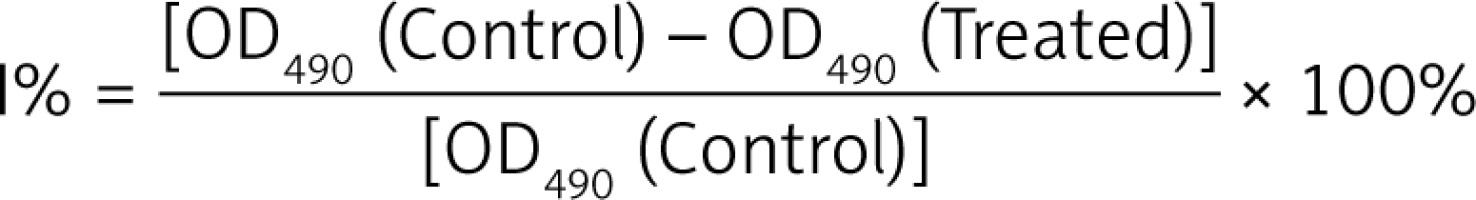Introduction
Gastrinoma arises as a result of a tumor in the duodenum or pancreas which secretes surplus amounts of gastrin, leading to ulcers in the stomach or duodenum. Gastrinoma mostly originates in the pancreas but can also be found in the duodenum or stomach. Pancreatic gastrinoma is characterized by a higher tendency to be malignant. Gastrin is a hormone that controls the amount of acid in the stomach. Gastrinomas produce enormous amounts of gastrin, and this causes the stomach to make more acid. Gastrinomas are a type of neuroendocrine tumors that develop in cells that are triggered by nerve cells to produce hormones. Gastrinomas are also known as pancreatic neuroendocrine tumors. Pancreatic neuroendocrine tumors (PNETs) are malignant tumors likely arising from islet cells of the pancreas [1, 2]. Pancreatic neuroendocrine tumors secrete a range of hormones including insulin, glucagon and somatostatin. However, several PNETs do not secrete any hormone [3]. Pancreatic neuroendocrine tumors can be very difficult to treat and can vary from benign to highly malignant. PNETs can be slow growing or aggressive in certain cases.
Regarding PNET treatment, surgical resection alone is mostly useful for its cure in initial stages. However, in most PNET cases (about 50%) patients have an advanced stage of the disease and then suffer from uncontrolled hormone secretion giving rise to many other complications [4]. It has been reported that gastrinoma tumors show a good response towards the use of cytotoxic chemotherapeutic drugs especially the combination of cisplatin and etoposide [5, 6]. However, there are serious side-effects associated with these cytotoxic anticancer drugs. Hence, there is a need to find alternative chemotherapeutic agents for the treatment of gastrinoma tumors. The objective of the present study was to evaluate the anticancer and apoptotic effects of dehydrocostus usually isolated from Cichorium intybus in the BON-1 PNET cell line and to explore the possible underlying mechanism.
Material and methods
Chemicals and other reagents used in this study
Dehydrocostus lactone (> 98%) was purchased from Chengdu Preferred Biotech Co. Ltd (China) and was dissolved in dimethyl sulfoxide (DMSO) at the concentration of 50 mM, stored as small aliquots at –20°C. Different concentrations of the compound were prepared (0, 5, 25, 50, 75 and 100 μM) for cell culture experiments. 3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl tetrazolium bromide (MTT) was purchased from Molecular Probes (USA). Dulbecco’s modified Eagle’s medium, fetal bovine serum (FBS), penicillin-streptomycin and rhodamine-123 (Rh-123) were obtained from Hangzhou Sijiqing Biological Products Co., Ltd, China. Propidium iodide (PI), trypsin, dimethyl sulfoxide (DMSO) and Hoechst 33258 were purchased from Sigma-Aldrich (USA).
Cell line and culture conditions
The human pancreatic neuroendocrine tumor cell line (BON-1) was procured from the Cancer Research Institute of Beijing, China. The cells were grown in DMEM supplemented with 10% fetal calf serum (FBS) and 150 U/ml of penicillin. Incubation of the cells was done at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The medium was stored at low temperature (2–5°C). The medium was replaced every 2 days. Cells were subcultured every 4 days.
Cell viability evaluation by MTT assay
The cytotoxic effects of dehydrocostus lactone on BON-1 cell proliferation were determined by MTT assays. BON-1 cells (2 × 105 cells/well) were seeded and cultured with varying doses of dehydrocostus lactone (0, 5, 25, 75, and 100 μM each) for 24 and 48 h. Following drug treatment, the medium was changed and 3-(4,5-dimethylthiazol-zyl)-2,5-diphenyltetrazolium bromide (MTT: 2 mg/ml) was added for 3 h. The number of viable cells is equal to the formation of formazan crystals which were dissolved in ethanol and the optical density was measured on a microplate reader (ELX 800; Bio-tek Instruments, Inc., Winooski, VT, USA) at a wavelength of 490 nm. The effects of dehydrocostus lactone on cell viability were calculated as an inhibition ratio (I %) using the following equation (optical density at 490 nm):
Detection of apoptosis using fluorescence microscopy
BON-1 cells (2 × 105 cells/well) were plated in six-well plates and then cultured for 24 h to allow complete attachment to the surface of the plates. The cells were treated with various doses of dehydrocostus lactone treatment (0, 5, 50 and 100 μM) for 48 h and then stained with Hoechst 33258 (2 μg/ml) at 37°C for 20 min. Nuclear morphology was examined under a fluorescence microscope (Olympus, Tokyo, Japan) to identify cells undergoing apoptosis.
Ultrastructural analysis by transmission electron microscope (TEM)
BON-1 cells were treated with or without dehydrocostus lactone for 48 h and fixed in sodium cacodylate (pH 7.4) and glutaraldehyde solution for 3 h. The cells were washed in PBS, then fixed in 1.5% OsO4 solution for 1 h at 25○C, washed and then dehydrated in an ethanol solution of increasing polarity (50% to 80% with 15 min of each bath). The cells were then embedded in EMbed 812 resin (SPI Supplies, PA, USA). After staining with uranyl acetate and lead citrate for 30 min, cell ultrastructure was analyzed (at 300 kV voltage) using ultra-thin sections with a transmission electron microscope (Hitachi High Technologies America, North 28th Avenue, Texas, United States).
DNA fragmentation analysis
BON-1 cells were seeded in a 100-mm cell culture dish for 48 h and treated with 0, 5, 50 and 100 μM dehydrocostus lactone for 48 h. The cells were harvested and washed with PBS, and the pellets were lysed with a 200 μl DNA lysis buffer (20 mM EDTA, 40 mM Tris-HCl) for 20 min. After centrifugation, the supernatants were prepared in an equal volume of 3% sodium-dodecyl sulfate, incubated with 3 mg/ml RNase A at 55°C for 3 h followed by digestion with 2.5 mg/ml proteinase K for 2 h at 20oC. Subsequent to the addition of 10 M ammonium acetate, the DNA was precipitated with cold ethanol and collected by centrifugation at 15,000 × g for 15 min. DNA was then dissolved in gel loading buffer, separated by electrophoresis in 1.5% agarose gel and visualized under UV light, following ethidium bromide staining.
Measurement of mitochondrial membrane potential (ΛΨm)
Mitochondrial membrane potential (MMP) in the human pancreatic neuroendocrine tumor cell line (BON-1) was measured with rhodamine-123 dye, which preferentially enters the active mitochondria based on the highly negative MMP. The cells were seeded in 96-well plates at a density of 1 × 105 cells/ml. The cells were treated with varying doses of dehydrocostus lactone (0, 5, 50 and 100 μM). Depolarization of MMP results in the loss of rhodamine 123 from mitochondria and a decrease in intracellular fluorescence is observed. Rhodamine 123 (final concentration of 10 μM) was added to the harvested cells and analyzed using a FACSCalibur instrument (BD Biosciences, San Jose, CA, USA) equipped with Cell Quest 3.3 software.
Cell cycle analysis
The effect of dehydrocostus lactone on cell cycle phase distribution was evaluated by flow cytometry using propidium iodide as a probe. BON-1 cells (2 × 105 cells/ml) were seeded in 60-mm dishes and treated with 0, 5, 50 and 100 μM of dehydrocostus lactone for 48 h. After treatment, the cells were trypsinized and washed twice with PBS. After that the cells were fixed with 70% cold ethanol overnight and then treated with 20 μg/ml RNase A, then stained with 3 μg/ml of propidium iodide. Finally the DNA content and cell cycle distribution were analyzed by flow cytometry. The experiments were repeated three times. The cell cycle analysis was performed by a FACSCalibur instrument (BD Biosciences, San Jose, CA, USA), equipped with Cell Quest 3.3 software with DNA propidium iodide (PI) staining.
Results
Antiproliferative effect of dehydrocostus lactone on the cytotoxicity of human pancreatic neuroendocrine tumor cell lines (BON-1)
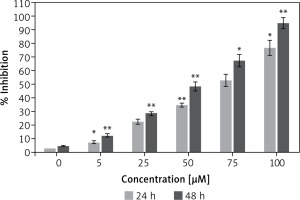
Figure 1 shows that the dehydrocostus lactone (Figure 2) exhibited dose-dependent as well as time-dependent growth inhibitory effects in these cells. The efficacy of the compound was evaluated by determining its IC50 value, which was found to be 71.9 μM and 52.3 μM at 24 and 48 h time intervals respectively.
Apoptotic effects of dehydrocostus lactone in BON-1 cells
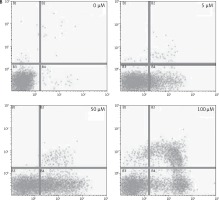
After treating BON-1 cells with 0, 5, 50 and 100 μM doses of the compound, the cells revealed apoptotic morphological features including nuclear fragmentation and condensation, cellular shrinkage, membrane blebbing, etc. In the untreated control group (Figure 3 A), most of the cells showed regular morphology and homogeneous growth and were confluent. However, after treating cells with 5, 50 and 100 μM doses of dehydrocostus lactone, apoptotic features described above began to appear and the effect intensified with increasing doses (Figure 3 A). The results were further confirmed by PI/annexin staining, which revealed that apoptotic cell populations increased from 6.79 at 5 μM to 48.5 % at 100 μM (Figure 3 B).
Figure 3
Apoptotic effect of indicated doses on dehydrocostus lactone in human pancreatic neuroendocrine tumor cell lines (BON-1). A – Fluorescence microscopy using Hoechst 33258 was carried out on treated and untreated cells. Apoptotic effect of indicated doses on dehydrocostus lactone in human pancreatic neuroendocrine tumor cell lines (BON-1). B – Annexin PI/annexin V staining analyzed by flow cytometry. All images are representatives of three biological replicates
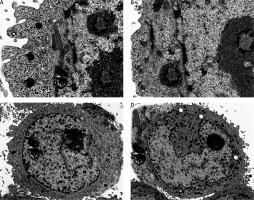
Ultrastructural analysis of cellular apoptosis by transmission electron microscopy
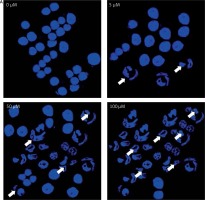
The fact that dehydrocostus lactone induces apoptosis in BON-1 cancer cells was further confirmed by using TEM. The results of this observation are shown in Figures 4 A–D. Control cells (untreated cells) exhibited normal ultrastructure with round nuclear membrane, normal mitochondria and the nucleolus present within the nucleus (Figure 4 A). But, after the cells were treated with 5, 50 and 100 μM concentrations of dehydrocostus lactone for 48 h, the mitochondria were destroyed, chromatin condensation occurred and the nuclear membrane disappeared. This was accompanied by the appearance of vacuoles in the cytoplasm, which is a hallmark of early apoptotic events which was clearly absent in untreated control cells (Figures 4 B–D). Thus TEM results confirm the results of fluorescence microscopy, which indicates that dehydrocostus lactone induces apoptosis in BON-1 cancer cells.
Figure 4
Transmission electron microscopy (TEM) analysis for evaluating the effect of dehydrocostus lactone on the apoptosis induction in BON-1 cells. A – Untreated control group, B–D –cells treated with 5, 50 and 100 μM dose of the compound (cells were observed at 8000× magnification). Appearance of vacuoles at higher doses is a sign of an early apoptotic event. All images are representatives of three biological replicates
Dehydrocostus lactone induces DNA fragmentation in BON-1 cells
DNA fragmentation analysis induced by different doses of dehydrocostus lactone was observed by the formation of a DNA ladder using 1.5% agarose gel electrophoresis. DNA ladder formation was consistent with increasing doses of the compound, but no such DNA laddering was visible in the control group (Figure 5). However, increasing doses of the compound for 48 h led to a significant increase in DNA fragmentation. The DNA fragmentation is a sign of the apoptotic process which starts within the cell, further confirming that the dehydrocostus lactone induced cell death via apoptosis.
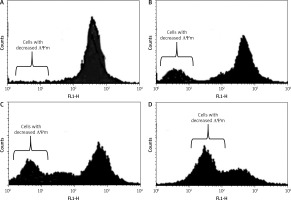
Dehydrocostus lactone induced mitochondrial membrane potential loss (ΛΨm loss)
Loss of mitochondrial transmembrane potential is a crucial step in the intrinsic apoptotic event. In this study, we evaluated the effect of dehydrocostus lactone on the mitochondrial membrane potential loss in BON-1 cells using a fluorescent probe, Rh-123 and flow cytometric analysis. After the drug treatment, a significant increase in cells with decreased membrane potential was seen after 48 h (Figures 6 A–D). The fraction of cells with decreased ΛΨm seemed to follow the concentration of the compound. The percentage of cells with decreased ΛΨm increased from 3.58% in the control group to 14.2%, 24.6% and 43.3% in 5, 50 and 100 μM-dehydrocostus lactone treated cells respectively. In cells, loss of ΛΨm leads to membrane rupture followed by cytochrome c release and pro-apoptotic factors.
Figure 6
Measurement of the effects of dehydrocostus lactone on the mitochondrial membrane potential loss (ΛΨm) in BON-1 cells using flow cytometry and Rh-123 as fluorescent probe. A – Untreated cells (control), while B–D – cells treated with 5, 50 and 100 μM doses of the compound. The percentage of cells with decreased ΛΨm increased from 3.58% in the control group to 14.2%, 24.6% and 43.3% in 5, 50 and 100 μM-dehydrocostus lactone treated cells respectively
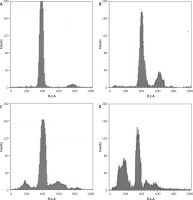
Dehydrocostus lactone induces sub-G1 cell cycle arrest in BON-1 cells
Figure 7 indicates that the compound increased the percentage of sub-G1 cells from 8.1% in the untreated group to 14.6%, 28.5% and 56.9% in groups treated with 5, 50 and 100 μM doses of dehydrocostus lactone respectively. The percentage of sub-G1 cells is an indication of the apoptotic cells which arise as a result of the treatment with the drug.
Figure 7
Dehydrocostus lactone induces sub-G1 cell cycle arrest in BON-1 cells. The cells were treated with different doses of dehydrocostus lactone (0 μM (A), 5 μM (B), 50 μM (C) and 100 μM(D)) and then analyzed by flow cytometry using propidium iodide as a probe. The flow cytometry images are representatives of three biological replicates
Discussion
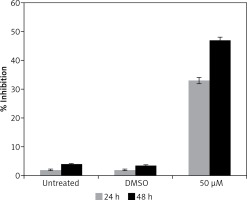
Gastrinoma is a very lethal type of cancer and is very difficult treat like, many other types of cancers. Moreover, treatment options for gastrinoma are limited and are often associated with numerous side effects [1]. The findings of this study indicate that dehydrocostus lactone is a potent cytotoxic agent which induces significant growth inhibitory effects in human pancreatic neuroendocrine tumor cell lines (BON-1) in a time-dependent as well as dose-dependent manner. Our studies are also consistent with previous studies wherein sesquiterpene lactones have been reported to exhibit anticancer activities against a range of cancer types [7]. It has also been reported that these lactones exhibit selective cytotoxic activity against cancer cells and exhibit high IC50 values for normal cell lines indicating lower cytotoxicity for normal cells [8]. Moreover, to evaluate the effect of the solvent, we determined the cytotoxic effects of DMSO on BON-1 and the results indicated that DMSO had negligible antiproliferative effects (Figure 8). Fluorescence microscopy using Hoechst 33258 showed that the compound induced apoptotic morphological features in these cells characterized by nuclear fragmentation and condensation, cellular shrinkage, membrane blebbing, etc. Further, transmission electron microscopy indicated that dehydrocostus lactone treatment at increasing doses led to formation of vacuoles, disappearance of nuclear membrane and damaged mitochondria. The effect intensified with increased doses of the compound. The untreated cells, however, showed normal cellular morphology with round nuclei and intact mitochondria. Further, using 1.5% agarose gel electrophoresis, DNA fragmentation analysis induced by dehydrocostus lactone was carried out. The results of this experiment indicated that as compared to the untreated control which did not show any signs of DNA fragmentation, dehydrocostus lactone treated cells at increasing doses indicated significant levels of DNA fragmentation. Consistent with this, several studies have reported induction of apoptosis by dehydrocostus lactone and costunolide in many types of cancer cells [7–9].
Figure 8
Effect of solvent DMSO on cell proliferation of BON-1 cells. The experiment was carried out in three biological replicates ± SD
Dehydrocostus lactone also induced significant loss of mitochondrial membrane potential (ΛΨm loss) in these cells. The percentage of cells with decreased ΛΨm seemed to follow the compound dose. The percentage of cells with decreased ΛΨm increased from 3.58% in the control group to 14.2%, 24.6% and 43.3% in 5, 50 and 100 μM-dehydrocostus lactone treated cells respectively. In cells, loss of ΛΨm leads to membrane rupture followed by cytochrome c release and pro-apoptotic factors. Loss of mitochondrial transmembrane potential is a crucial step in the intrinsic apoptotic event [10, 11]. This compound also led to sub-G1 cell cycle arrest in BON-1 cells, the percentage of sub-G1 cells increasing from 8.1% in the untreated group to 14.6%, 28.5% and 56.9% in groups treated with 5, 50 and 100 μM doses of dehydrocostus lactone respectively.
It has been reported that the process of cancer development is closely linked to the biochemical processes of cell cycle and apoptosis. Currently, the drug discovery process focuses on chemotherapeutic agents which can target the cell cycle and apoptosis [9–14].
Dehydrocostus lactone belongs to the sesquiterpene lactone class of naturally occurring compounds. They are sesquiterpenoids and contain a lactone ring. Sesquiterpene lactones are found in several plants and are well known for causing allergic reactions [15, 16]. Dehydrocostus lactone along with costunolide has been reported to induce apoptosis and cell cycle arrest in human soft tissue sarcoma cells. Dehydrocostus lactone caused sub-G1 cell cycle arrest, as evident from the increased number of sub-G1 cells in the treated cells. A previous study reported that a similar type of compound causes G2/M cell cycle arrest [17, 18]. Moreover, the current study used a different and rare cancer cell line, viz. BON-1 (human pancreatic neuroendocrine tumor cell lines), which may be one reason for the cell cycle results reported in the present study. The findings of the present study report a different mechanism of action of dehydrocostus lactone. It induced sub-G1 cell cycle arrest and also induced loss of mitochondrial membrane potential, indicating the intrinsic apoptotic pathway.
In conclusion, the findings of the present study reveal that dehydrocostus lactone inhibited cancer cell growth in human pancreatic neuroendocrine tumor cell lines via the induction of mitochondrial mediated apoptosis, cell cycle arrest, DNA fragmentation and loss of mitochondrial membrane potential.