Introduction
Drug-induced hypersensitivity syndrome (DiHS) or drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a rare and severe hypersensitivity reaction to a drug that includes skin eruption, hematologic abnormalities (eosinophilia, atypical lymphocytes), lymphadenopathy, and internal organ involvement (liver, kidney, lung, etc.) [1, 2]. DiHS/DRESS syndrome is characterized by a long latency (2 to 8 weeks) after the initiation of drug therapy and the possible persistence or aggravation of symptoms despite the discontinuation of the culprit drug, and frequent association with the reactivation of a latent human herpes virus (HHV) or other infection [3]. The pathogenesis of DiHS/DRESS syndrome is understood partially. Different mechanisms have been implicated in its development, including genetic susceptibility associated with human leucocyte antigen (HLA) loci, detoxification defects leading to reactive metabolite formation and subsequent immunological reactions, slow acetylation, and reactivation of human herpes, including Epstein-Barr virus and HHV-6 and HHV-7 [4, 5]. We report a case of DiHS/DRESS induced by second-line treatment for tuberculosis and Epstein-Barr virus re-infection.
Case report
A 31-year-old woman was admitted to the hospital for the treatment of newly diagnosed drug-resistant (pre-extensively drug resistant) pulmonary tuberculosis (chest X-ray at time of diagnosis – Fig. 1). She was treated with second-line antituberculosis drugs: moxifloxacin, kanamycin, cycloserine, prothionamide, para-aminosalicylic acid. After 3 weeks of therapy she developed high fever (> 39°C), lymphadenopathy in the cervical and axillary regions and pruritic maculopapular eruption all over the body (Fig. 2). Hematologic abnormalities such as leukocytosis with eosinophilia (1.81 × 109/l) and monocytosis (1.85 × 109/l) were detected in peripheral blood of the patient. Hepatitis was asymptomatic and detected by the evaluation of liver function: serum aspartate aminotransferase (AST) 1379 IU/l and alanine aminotransferase (ALT) 1221 IU/l; levels of liver enzymes were increased by approximately 30-40-fold above the normal limits. The positive diagnosis of Epstein-Barr infection was based on the onset of increase in the anti-Epstein-Barr immunoglobulin (Ig) G titer (> 200 U/ml), implicating Epstein-Barr virus re-activation. Based on the clinic and laboratory findings diagnosis of DiHS/DRESS was suspected, and all the drugs were discontinued. Symptoms and laboratory abnormalities gradually resolved over 4 weeks without additional treatment.
Fig. 1
Chest X ray posterior-anterior view (A) and right lateral view (B) on admission. On the superior lobe posterior segment of the right lung a mild parenchymal opacity with several nodules can be seen
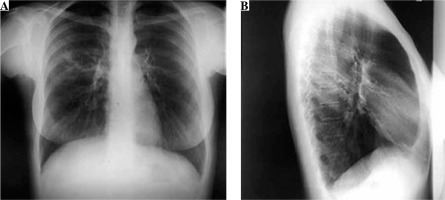
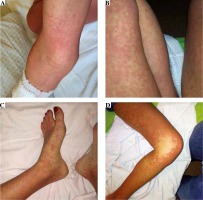
Fig. 2
Macculopapular exanthema on the patients body after three weeks of second-line treatment for tuberculosis
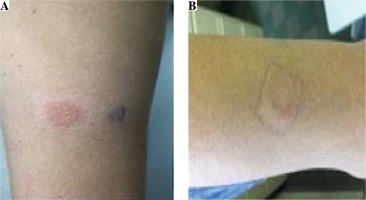
A patch test was performed [6] and analyzed [7] according to the recommendations. The results of the patch test are presented in Table 1 and Figure 3. Treatment was adjusted to ethambutol, kanamycin, cycloserine, pyrazinamide, and linezolid, and no new symptoms were observed.
Discussion
DiHS/DRESS syndrome is a severe adverse reaction to a drug characterized by various symptoms – skin rash, fever, lymph node enlargement and internal organ involvement – which starts within 2 weeks to 3 months after drug initiation. The pathogenesis of DiHS/DRESS is not fully understood, but it is associated with a delayed immunological reaction to a drug [1, 8]. Genetic polymorphism (HLA) and HHV reactivation may also cause DiHS/DRESS syndrome. The death rate is about 10%, mostly due to liver damage thought to be mediated by infiltration of eosinophils [9]. The most frequently reported causes of DiHS/DRESS are antiepileptic agents, allopurinol, sulfonamides and dapsone [10]. Anti-tuberculosis drugs may also induce DiHS/DRESS syndrome [11]; according to the literature, rifampicin was the most commonly suspected drug, followed by isoniazid, ethambutol and pyrazinamide [12].
PubMed search showed only a few case reports of DiHS/DRESS induced by the second line anti-tuberculosis drugs, such as prothionamide and para-aminosalicylic acid [13]. The drug provocation test is generally accepted as a gold standard for confirmation of the culprit drug [14] and may minimize anti-tuberculosis treatment interruption; however, in severe cases, such as the currently described case, the provocation test is not ethically recommended. Furthermore, in a case which involves multiple complexed treatment, the patch test may be a useful method for detection of the culprit drug, as we applied in our case. According to the guidelines for performing patch tests in drug-induced cutaneous adverse reactions, tests should be performed 6 months after the complete healing of DiHS/DRESS [6, 15]. However, previous patch tests could be justified because of the nature of a tuberculosis infection, the lack of adequate therapeutic alternatives, and the risk/benefit balance. A positive test result is highly dependent on the causative drug. We tested all suspected drugs incorporated at 30% in white petrolatum (pet.) [6]. Our patient’s patch test was positive to prothionamide and para-aminosalicylic acid. The analysis of patch test results was difficult because it was performed relatively close to the acute phase and in the context of immunosuppression due to tuberculosis infection. According to the ENDA/EAACI Drug Allergy Interest Group position paper, it might be advisable to perform the lymphocyte transformation test (LTT) before in vivo tests in severe reactions with a suspected T cell mechanism [16]. However, positive LTT reactions can only be obtained at the recovery phase, 2 months after onset; thus negative LTT reactions at the acute phase could alternatively be interpreted as suggesting a diagnosis of DiHS/DRESS [17].
Recently, Epstein-Barr virus, HHV-6, and HHV-7 reactivations were found in 76% of DiHS/DRESS cases in a clinical study. Interestingly, the culprit drugs were able to trigger viral reactivations that induce a pathogenic antiviral T cell immune response [18]. In our case, Epstein-Barr laboratory tests showed IgG antibody positive reaction, but the PCR assay to detect the virus was not done. Hence an association of DiHS/DRESS with Epstein-Barr virus infection cannot be fully confirmed.
In conclusion, the diagnosis of DiHS/DRESS should be highly suspected with the presence of skin rash, liver involvement, fever, eosinophilia, and lymphadenopathy. The list of drugs causing DiHS/DRESS syndrome should be expanded with second-line drugs for tuberculosis treatment such as prothionamide and para-aminosalicylic acid. The high rate of herpes viruses reactivation associated with DiHS/DRESS implies that these viruses should be detected in all suspected cases during daily clinical practice. Additionally, clinicians who prescribe treatment for tuberculosis must be aware of the possibility of a severe hypersensitivity reaction to anti-tuberculosis drugs.