Introduction
Prostate cancer (PCa) is the among the most common cancer types among men and a common cause of cancer-related deaths [1]. Imaging plays a role in the staging of PCa after confirmation of the histopathological diagnosis. Magnetic resonance imaging (MRI) was first used for prostate gland imaging in the early 1990s [2]. Multiparametric MRI (mpMRI) has been shown to increase cancer detection and evaluation of extracapsular extension [3]. mpMRI refers to assessment of the prostate gland using T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and dynamic contrast-enhanced (DCE) MRI with or without magnetic resonance proton spectroscopy (MRS). mpMRI also helps detect pelvic nodal metastases and bone metastasis with high sensitivity and specificity.
In 2015, the Prostate Imaging Reporting and Data System (PI-RADS) guidelines document was designed to simplify the steps of prostate mpMRI, from acquisition to reporting [4]. Although PI-RADS version 2.1 enlists the minimum acceptable technical standards for mpMRI of the prostate gland, the equipment still varies considerably across institutions in the country [5,6]. In particular, the use of magnet strengths (3.0 T vs. 1.5 T) for prostate MRI is not uniform (Figure 1). PI-RADS states that both 1.5 T and 3.0 T can provide adequate and reliable diagnostic examinations. However, most members of the PI-RADS Steering Committee prefer, use, and recommend 3.0 T for prostate MRI [6]. The American Urological Association prostate imaging standard operating procedure mentions that prostate MRI can be obtained with a conventional 1.5 T or 3.0 T magnet with or without use of an endorectal coil [7]. No specific magnet strength for MRI for PCa imaging is mentioned by the European Association of Urology–European Society for Radiotherapy and Oncology–International Society of Geriatric Oncology guidelines [8], the American College of Radiology Appropriateness Criteria [9], or the National Comprehensive Cancer Network guidelines on imaging of PCa [10].
Figure 1
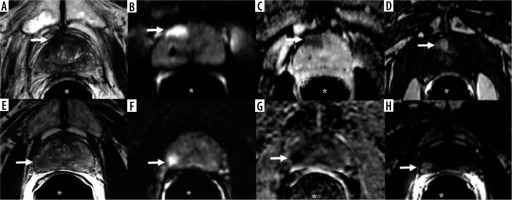
A-D) A 78-year-old man with prostate adenocarcinoma, Gleason score 6 (3 + 3). A) Axial T2-weighted imaging, (B) diffusion-weighted image (b = 800 s/mm2), (C) apparent diffusion coefficient, and (D) post-contrast T1-weighted imaging of 1.5 T MRI show a 1.5 × 0.9 cm dominant lesion in the right peripheral zone at the level of the mid-gland near the apex (arrow) with low T2 signal, restricted diffusion, and post-contrast enhancement. Qualitative suspicion of clinically significant disease: 5. E-H) A 59-year-old man with prostate adenocarcinoma, Gleason score 7 (4 + 3). E) Axial T2-weighted imaging, (F) diffusion-weighted image (b = 800 s/mm2), (G) apparent diffusion coefficient, and (H) post-contrast T1-weighted imaging of 3 T MRI show a 1.1 × 1.0 cm dominant lesion located in the right peripheral zone at the level of the mid-gland (arrow) with low T2 signal, restricted diffusion, and post-contrast enhancement. Qualitative suspicion of clinically significant disease: 5. *Endorectal coil
Considering this uncertainty, there is a need for a systematic analysis of the existing evidence to determine the optimal MRI magnet strength for PCa imaging. Few studies have compared the diagnostic value of 3.0 T with that of 1.5 T MRI for the staging of PCa, and to our knowledge, there are no meta-analyses on this topic. Accordingly, the aim of this systematic review and meta-analysis was to compare the diagnostic performance of 3.0 T with that of 1.5 T MRI in the staging of PCa.
Material and methods
Search strategy
In this study, we followed the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines [11]. We searched all available literature published through May 2020 in MEDLINE, Embase, and the Cochrane Database of Systematic Reviews. The databases were comprehensively searched using the following keywords: “1.5 T MRI” or “1.5 T magnetic resonance imaging” AND “3.0 T MRI” or “3.0 T magnetic resonance imaging” AND “prostate” or “prostatic” AND “cancer” or “carcinoma” or “neoplasm”.
The reference lists of all retrieved studies were scrutinized to identify additional articles to supplement the search results.
Inclusion criteria
Studies were eligible for inclusion if all the following applied: (a) the diagnostic performances of 3.0 T and 1.5 T MRI in PCa were clearly identified in the study; (b) the numbers of true-positive, true-negative, false-positive, and false-negative results could be obtained from the article; and (c) the reference standard for malignancy was histopathological analysis, imaging, or both.
Exclusion criteria
We excluded the following kinds of articles: (a) studies not published in English; (b) review articles, meeting abstracts, letters, and case reports; and (c) articles without sufficient data to construct a 2 × 2 contingency table.
Study quality assessment
After the literature search and the application of inclusion and exclusion criteria, a single reviewer (M.V.) assessed the quality of all eligible studies using the current Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 tool [12]. This tool includes 4 major domains: patient selection, index test, reference standard, and flow and timing. These domains were then further assessed based on the risk of bias, and their applicability was rated as “high”, “low”, or “unclear”. A second reviewer (P.B.) assessed the accuracy of this assessment.
Data extraction
The selected articles were reviewed, and key information (e.g. study design, technical specifications) was collected. Any disagreements regarding the interpretation of data were resolved by consensus of the reviewers. The location of the primary tumour and its size were not available in all the studies and were not selected for analyses. The numbers of true-positive, true-negative, false-positive, and false-negative results were obtained or derived from the studies.
Diagnostic performance analysis
To reduce clinical heterogeneity (in pre-test probability of malignancy) and methodological heterogeneity, our primary diagnostic performance analysis for 3.0 T and 1.5 T MRI included only studies in which both magnet strengths were used.
Reference standard
The reference standard comprised histological confirmation of the lesion (obtained during surgery or biopsy) and/or imaging findings. In the metanalysis, 3 studies used histological confirmation as the reference standard. In the article by Park et al. [13], both the histological confirmation and imaging served as the reference standard.
Statistical analysis
Data from published studies involving both 1.5 T and 3.0 T MRI in patients with PCa were collected from the literature. Studies by Beyersdorff et al. [14] and Torricelli et al. [15] were included; each had information for a 1.5 T group and a separate 3.0 T group. In a study by Park et al. [13], positivity was analysed separately for tumour stage, extracapsular extension, seminal vesicle involvement, and either extracapsular extension or seminal vesicle involvement, with the same patients in each analysis. Of these analyses, the tumour stage analysis was chosen for data extraction because those data were the most relevant to the other selected articles. A study by Ryznarova et al. [16] had 3 patient groups: Group A (1.5 T), Group B (3.0 T standard), and Group C (3.0 T without dynamic contrast enhancement). Groups B and C were combined into a single 3.0 T group for our analysis.
For each study group (1.5 T or 3.0 T), we categorized results into true positives, true negatives, false positives, and false negatives. In cases where there were 0 results in at least one of these 4 categories, a correction factor of 0.5 was added to the number of results in each cate-gory to make all calculations finite. Sensitivity, specifi-city, and diagnostic odds ratio (DOR) were first calculated for each study group. Summary tables were then used to summarize the results across studies. The pooled sensitivity, specificity, and DOR for each magnet strength were estimated from a random-effects model using the Der-Simonian and Laird approach [17]. Summary receiver operating characteristic figures were used to summarize the relationship between sensitivity and specificity, and the area under the receiver operating characteristic curve (AUC) was provided as a summary measure for each magnet. Study heterogeneity was assessed with Cochran’s Q test and the Higgins I2 statistic. A Deeks’ funnel plot was used to assess publication bias. All statistical analyses were performed using R version 3.6.1. All statistical tests used a significance level of 5%. No adjustments for multiple testing were made.
Results
Study selection and description
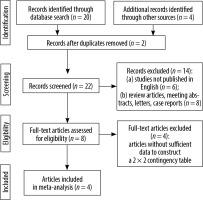
The initial search yielded 24 articles (Figure 2), of which 8 were reviewed. Of these, 4 studies involved both 3.0 T and 1.5 T MRI in patients with PCa and were thus eligible for the study. The characteristics of the included studies are summarized in Table 1.
Table 1
Characteristics of four studies with 3.0 T and 1.5 T MRI for diagnosis of prostate cancer
| Author | Number | 3.0 T scanner | 3.0 T MRI sequences | 1.5 T scanner | 1.5 T MRI sequences | Objective | Subjects and primary findings |
|---|---|---|---|---|---|---|---|
| Beyersdorff [14] | 22 | Signa 3.0 T (GE Healthcare) using a torso phased-array coil (USA Instruments) | After a localizer scan, at least a T2W angulated axial FSE sequence with a TR/TE of 4,500/102 and an ETL of 8 or 16 and an angulated coronal FSE sequence (3,800/78.3; ETL, 8) with a FOV of 16 × 16 cm and a matrix of 256 × 256 were acquired. Four acquisitions were performed with each sequence at a slice thickness of 4 mm and a gap of 1 mm*. | Magnetom Vision or Magnetom Symphony (Siemens Medical Solutions) using a combination of an endorectal coil (Medrad) and a body phased-array coil. | T2W TSE sequence in angulated axial (3,500/96; ETL, 13; 3 acquisitions) and coronal (4,522/112; ETL, 13; 2 acquisitions) slice orientations with a FOV of 16 × 16 cm. The matrix was 256 × 256. In addition, an angulated axial T1W spin-echo sequence (680/14; ETL, 3) was acquired. The FOV was 16 × 16 cm, and the matrix was 256 × 256. The slice thickness was 3 mm and the interslice gap, 0.9 mm*. | To compare the image quality, tumour delineation, and depiction of staging criteria on MRI of PCa at 1.5 and 3.0 T. | No statistically significant differences in the visualization of staging criteria. |
| Torricelli [15] | 29 | Intera 3.0 T magnet, operating at 3.0 T (Philips Medical System), using a 6-channel external phased-array cardiac receiver coil | TSE T2W (TR/TE: 5504/120 ms) in the axial and coronal plane with ETL: 21, NSA: 4, FOV: 210 mm, slice thickness: 3 mm, gap: 0.5 mm, scan matrix: 320 × 320, and scan reconstruction: 512 × 512. TSE T1w (TR/TE: 445/11 ms) in the axial plane with ETL: 3, NSA: 2, FOV: 210 mm, slice thickness: 3 mm, gap: 0.5 mm, scan matrix: 320 × 320, and scan reconstruction: 512 × 512. | Intera 1.5 T Philips magnet, operating at 1.5 T (Philips Medical System), using an Ecca 64 MHz endocavitary coil (Philips Medical System). | TSE T2W (TR/ TE: 4750/130 ms) in the axial and coronal plane with ETL: 18, NSA: 6, FOV: 180 mm, slice thickness: 3 mm, gap: 0.5 mm, scan matrix: 320 × 320, and reconstruction matrix: 512 × 512. TSE T1W (TR/TE: 445/11 ms) in the axial plane with ETL: 3, NSA: 2, FOV: 180 mm, slice thickness: 3 mm, gap: 0.5 mm, scan matrix: 320 × 320, and reconstruction matrix: 512 × 512. | To compare the image quality and the diagnostic accuracy of endorectal coil 1.5 T MRI and phased-array coil 3.0 T MRI in staging of PCa. | 3.0 T MRI had worse image quality but can provide similar diagnostic information compared with 1.5 T MRI. |
| Park [13] | 108 | Intera Achieva 3.0 T (Philips Medical System); examination was performed using a 6-channel external phased-array cardiac receiver coil (USA Instruments) | TSE using a SENSE technique (factor = 2) was used for T2W imaging with the following parameters: TR, 3300 to 4000 ms; TE, 80 to 100 ms; TSE factor, 12; FOV, 15 cm (17 cm for sagittal images); matrix number, 304 × 304; one acquisition; slice thickness, 3 mm; gap, 0.3 mm. Second, axial T1W imaging in a large FOV (24 cm) at both 3.0 and 1.5 T.** | Genesis Signa; GE Healthcare with endorectal coil (Medrad). | FSE was used for T2W imaging with the following parameters: TR, 3300-4000 ms; TE, 80-100 ms; ETL, 13; FOV, 18 cm; matrix number, 512 × 256; one acquisition; slice thickness, 3 mm; gap, 1 mm. Second, axial T1W imaging in a large FOV (24 cm) at both 3.0 and 1.5 T.** | To evaluate local staging accuracy for PCa at 3.0 T MRI compared with 1.5 T MRI. | 3.0 T phased-array MRI is equivalent to the 1.5 T endorectal MRI in evaluating local staging accuracy for PCa without significant loss of imaging quality. |
| Ryznarova [16] | 103 (1.5 T: 41, 3 T: 63) | Trio, Siemens, using 8-channel phased-array surface coils. | T1W TSE sequence in axial plane T2W TS sequences were performed in axial (orthogonal to the urethra), coronal, and sagittal planes. Echo-planar DWI was obtained in transverse plane parallel to the transverse T2W to construct ADC maps using the standard Siemens software. DCE 3D T1-spoiled gradient echo images were acquired during an intravenous bolus injection of paramagnetic contrast medium (gadobenate dimeglumine) at a dose of 0.1 mmol/kg of body weight for examination at 3.0 T. A MRS was also obtained by using a point-resolved 3D spectroscopic imaging sequence. | Avanto Siemens, Erlangen, Germany using 8 channels phased-array surface coils. | T1W TSE sequence in axial plane; T2W TSE sequences were performed in axial (orthogonal to the urethra), coronal, and sagittal planes. Echo-planar DWI was obtained in transverse plane parallel to the transverse T2W to construct ADC maps using the standard Siemens software. DCE 3D T1-spoiled gradient echo images were acquired during an intravenous bolus injection of paramagnetic contrast medium (gadobenate dimeglumine) at a dose of 0.2 mmol/kg of body weight for examination at 1.5 T. MRS was also obtained by using a point-resolved 3D spectroscopic imaging sequence. | To compares the results of MRI obtained by the 1.5 T and 3.0 T scanners using surface coils in patients with PCa. | Highest accuracy of local PCa staging with 3.0 T MRI scanner was seen when the protocol included DCE. No significant difference was found in tumour localization assessment between 3.0 T and 1.5 T MRI scanners. |
MRI – magnetic resonance imaging, PSA – prostate-specific antigen, P – prospective, R – retrospective, TSE – turbo spin echo, FSE – fast spin echo, T2W – T2-weighted, T1W – T1-weighted, ETL – echo train length, TE – echo time, TR – repetition time, FOV – field of view, PCa – prostate cancer, NSL – number of signals averaged, SENSE – SENSitivity Encoding, DWI – diffusion-weighted imaging, DCE – dynamic contrast enhancement, ADC – apparent diffusion coefficient, MRS – proton magnetic resonance spectroscopy
QUADAS-2 assessment
The methodologic results of the QUADAS-2 assessment are presented in Table 2 and Figure 3. In all studies, we found a low risk of bias with regard to participant selection, except in Park et al. [13]. In their study, 20 patients were excluded because their 1.5 T MRI was performed at an outside local hospital and had poor image quality. In addition, 11 patients with prostatectomy were excluded because of highly deviated age, prostate-specific antigen level, and Gleason scores. There was a low risk of bias in reference standards in all studies. We made this determination for the reference standards because the included studies used histopathological confirmation, imaging, or both to validate patients’ diagnoses. For the index test domain, studies had a low risk of bias because of blinding to the reference test. Most studies could be evaluated in terms of the low risk of bias in flow and timing because the studies provided the interval between the index test (MRI) and the reference standard (histopathological confirmation and/or imaging follow-up), except for the study by Torricelli et al. [15].
Figure 3
Methodologic quality of all eligible studies according to the domains of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 tool
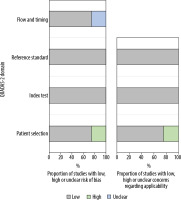
Table 2
Tabular presentation of Quality Assessment of Diagnostic Accuracy Studies 2 results
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Beyersdorff [14] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ |
| Torricelli [15] | ☺ | ☺ | ☺ | ? | ☺ | ☺ | ☺ |
| Park [13] | ☹ | ☺ | ☺ | ☺ | ☹ | ☺ | ☺ |
| Ryznarova [16] | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ |
Diagnostic performance of 3.0 T and 1.5 T MRI
3.0 T MRI analysis
For 3.0 T, the pooled sensitivity was 69.5%, with a 95% CI of 56.4-80.1%. The pooled specificity for 3.0 T was 48.8%, with a 95% CI of 6-93.4%. The pooled DOR for 3.0 T was 3, with a 95% confidence interval of 0-26. The AUC was 0.684. As described above, 2 assessments of heterogeneity among studies were performed for DOR: Cochran’s Q was 3.4 (p = 0.33), and the Higgins I2 was 12.1, indicating no evidence of heterogeneity. There was also no evidence of publication bias (p = 0.83) on the Deeks’ funnel plot.
1.5 T MRI analysis
The pooled sensitivity for 1.5 T was 70.6%, with a 95% CI of 55.0-82.5%. The pooled specificity for 1.5 T was 41.7%, with a 95% CI of 6.2-88.6%. The pooled DOR for 1.5 T was 2, with a 95% confidence interval of 0-18. The AUC was 0.679. For DOR, 2 assessments of heterogeneity among studies were performed: Cochran’s Q was 3.5 (p = 0.32), and the Higgins I2 was 15.3, indicating no evidence of heterogeneity. There was also no evidence of publication bias (p = 0.88) on the Deeks’ funnel plot.
Comparison of 3.0 T and 1.5 T MRI
In the meta-analysis, we compared 3.0 T and 1.5 T MRI for staging of PCa and found no differences between the magnet strengths in sensitivity, specificity, or DOR values (p = 0.91, 0.88, and 0.89, respectively). Table 3 compares the diagnostic performances of the 2 magnets. Figures 4 and 5 show summary receiver operating characteristic curves for the 3.0 T and 1.5 T MRI, respectively.
Figure 4
Summary receiver operating characteristic curve for 3.0 T. Red diamond represents pooled sensitivity and specificity, while surrounding blue region represents the 95% confidence region around this estimate. Green triangles represent individual study estimates. The area under the summary receiver operating characteristic curve was 0.842
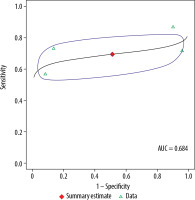
Figure 5
Summary receiver operating characteristic curve for 1.5 T. Red diamond represents pooled sensitivity and specificity, while the surrounding blue region represents the 95% confidence region around this estimate. Green triangles represent individual study estimates. The area under the summary receiver operating characteristic curve was 0.845
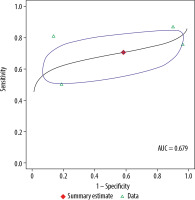
Table 3
Diagnostic performances of 3.0 T vs. 1.5 T MRI in staging of prostate cancer
[i] AUC – area under the summary receiver operating characteristic curve, CI – confidence interval, CT – computed tomography, DOR – diagnostic odds ratio, FN – false negative, FP – false positive, MRI – magnetic resonance imaging, TN – true negative, TP – true positive. A p-value of ≤ 0.05 was considered statistically significant.
Discussion
In our meta-analyses, we found no statistically significant differences between 3.0 T and 1.5 T MRI in sensitivity, specificity, or DOR values in the evaluation of PCa. Individual comparisons of sensitivity and specificity in the meta-analysis were limited by different thresholds used by the included studies. Our DOR results indicate a potentially higher diagnostic accuracy of 3.0 T compared with 1.5 T MRI (3 vs. 2, respectively); however, these differences were not statistically significant (p = 0.89).
The literature contains discordant results on the diagnostic accuracy of 3.0 T and 1.5 T MRI in PCa. In consensus with our study, Ullrich et al. reported similar PI-RADS scoring between 1.5 T and 3.0 T MRI without the use of an endorectal coil [18]. In their study, all 63 patients (64 ± 9 years of age) received mpMRI (T2WI, DWI, and DCE) on a 3.0 T scanner and bi-parametric MRI (T2WI and DWI) on a 1.5 T scanner using body coils. The signal-to-noise ratio and contrast-to-noise ratio of T2WI were similar between 1.5 T and 3.0 T (p = 0.7-1), but for DWI these ratios were significantly lower at 1.5 T (p < 0.01). PI-RADS scores were similar between 1.5 T and 3.0 T MRI (p = 0.05-1). The authors concluded that the diagnostic performance is independent of the magnet strengths even without an endorectal coil. Similarly, Shah et al reported that non-endorectal coil 3.0 T MRI provides similar image quality to that obtained with endorectal coil 1.5 T MRI in PCa [19]. In this study of 83 patients, 2 readers graded the image quality on axial and coronal T2WI fast spin echo (FSE). For both readers, the endorectal coil 1.5 T MRI showed similar accuracy to non-endorectal coil 3.0 T MRI for PCa localization (Reader 1: 0.5664 for 1.5 T and 0.5521 for 3.0 T, p = 0.701; Reader 2: 0.6095 for 1.5 T and 0.5932 for 3.0 T, p = 0.628), with non-consensus results for extra-prostatic extension and seminal vesicle involvement.
In addition, a study of 108 patients reported an accuracy of 72% (39/54) for 3.0 T phased-array MRI and 70% (38/54) for endorectal coil 1.5 T MRI for T3 staging of PCa (p = 0.05) [13]. Apart from the lower incidence of MRI artifacts on 3.0 T MRI (p = 0.00), the overall imaging quality did not significantly differ between 3.0 and 1.5 T (31% vs. 41%, respectively, p = 0.05). The 3.0 T MRI protocol for the study consisted of triplanar T2WI turbo spin echo (TSE), while T2WI FSE was used for 1.5 T MRI, and axial T1WI was included for both protocols. Sosna et al. reported that 3.0 T with a large field of view (25 cm; n = 14) produced an image of similar quality to that of 1.5 T (n = 20) for visualization of the posterior border (p = 0.3893), seminal vesicles (p = 0.8680), neurovascular bundles (p = 0.2684), and overall image quality rating (p = 0.8599) using triplanar T2WI FSE MRI sequences [20].Ye et al. reported that 3.0 T MRI had improved performance compared with 1.5 T MRI in detecting local PCa; however, the difference was not statistically significant (p > 0.05) [21]. 1.5 T MRI had a sensitivity of 0.82-0.87, a specificity of 0.12-0.2, and an accuracy of 0.51-0.51, while 3.0 T MRI had a sensitivity of 0.89-0.91, a specificity of 0.24-0.28, and an accuracy of 0.51-0.65. The study reported a high number of false positives (specificity of 0.26 for 3.0 T vs. 0.16 for 1.5 T) and interpreted only T2WI of the peripheral zone of the prostate.
The addition of the DCE MRI technique has shown no significant change in diagnostic performance of the 3.0 T and 1.5 T MRI in PCa imaging. Sertdemir et al. studied the diagnostic potential of the DCE MRI parameters of plasma flow and mean transit time for differentiating prostate carcinoma and normal prostate tissue on 1.5 T and 3.0 T MRI [22]. The authors used triplanar T2WI TSE, axial echo planar DWI, 3D chemical shift imaging MRS, and axial DCE 2D TurboFLASH MRI sequences with an endorectal coil. They reported no significant relationship between Gleason score and either plasma flow or mean transit time for 1.5 T (p = 0.17 and 0.11, respectively) or for 3.0 T MRI (p = 0.23 and 0.18, respectively). In agreement with the preceding study, Ryznarova et al. also reported no statistically significant difference in sensitivity or specificity of local carcinoma staging between 1.5 T and 3.0 T scanners [16]. In the 1.5 T MRI group, for tumours localized inside the prostate (T2 stage), the sensitivity and specificity were 72% and 56%, respectively; for extracapsular tumour extension (T3a), 50% and 83%, respectively; and for seminal vesicle infiltration (T3b), 75% and 95%, respectively. In the 3.0 T MRI group, the sensitivity and specificity were 100% and 77%, respectively, with DCE, and 83% and 57%, respectively, without DCE for T2 stage prediction; 70% and 100%, respectively, with DCE, and 46% and 86%, respectively, without DCE for T3a stage prediction; and 100% in all subgroups for T3b stage prediction. The overall accuracy in tumour stage prediction was 66% for 1.5 T, 90% for 3.0 T with DCE, and 72% for 3.0 T without DCE, and it did not significantly differ between these magnets in the assessment of tumour localisation. The study MRI protocol included axial T1W TSE, triplanar T2W TSE, DCE 3D T1-spoiled gradient echo, and proton MRS.
Contrary to our study, Torricelli et al. reported that 1.5 T MRI image quality was significantly better than that of 3.0 T MRI in the staging of PCa by the 2 readers with respect to tumour conspicuity (p = 0.0175, 0.0038), prostate capsule (p = 0.0042, 0.0009), and seminal vesicles (p = 0.0003, 0.0003) [15]. There was no significant difference in the scores applied to the apex (p = 0.779, 0.3293) and neurovascular bundles (p = 0.4035, 0.6801). The MRI sequences included axial and coronal T2WI TSE and axial T1WI TSE using a phased-array cardiac receiver coil (3.0 T) and an endorectal coil (1.5 T). Another study, by Nieuwenhove et al., prospectively compared the diagnostic performance of a biparametric 1.5 T MRI (T2WI and DWI) with 3.0 T mpMRI in patients referred for a prostate biopsy [23]. The 1.5 T MRI protocol consisted of triplanar T2WI and axial DWI without an endorectal coil, and the 3.0 T MRI consisted of triplanar T2WI TSE, axial DWI, and axial DCE imaging. The authors reported a high diagnostic accuracy for 1.5 T MRI, using mpMRI as the reference standard, with a per-patient AUC of 0.975 and a per-lesion AUC of 0.961 (p < 0.001). The study also reported that in high-risk PCa patients undergoing prostate biopsy, a 1.5 T MRI (T2WI + DWI) protocol can be used instead of a 3.0 T mpMRI protocol, saving time and contrast injection. In agreement with the preceding studies, Beyersdorff et al. [14] reported significantly better scores for the images obtained at 1.5 T than for those obtained at 3.0 T MRI for overall image quality (p < 0.001) and the criteria of delineation of the prostate capsule (p = 0.007), depiction of the zonal anatomy (p = 0.003), and delineation of the tumour (p = 0.012). Artifacts were seen at both magnet strengths; however, there were fewer artifacts at 1.5 T, which were caused by the endorectal coil, while motion artifacts and technical artifacts were seen at 3.0 T (p = 0.002). The study used axial and coronal T2WI FSE sequences for 3.0 T MRI and axial and coronal T2WI TSE and axial T2WI SE sequences for 1.5 T MRI with an endorectal coil and a body phased-array coil.
The effect of MRI magnet strength has also been studied in the field of prostate imaging research. Heesakkers et al. conducted a prospective study for feasibility of ferumoxtran-10-enhanced MRI at 3.0 T and to compare image quality between 1.5 T and 3.0 T MRI in lymph node detection in patients with PCa [24]. Ferumoxtran-10 is a synthetic ultrasmall superparamagnetic iron oxide, composed of dextran-coated iron oxide nanoparticles (also known as ultrasmall particulate iron oxides), which accumulates in non-cancerous lymphatic tissue and is used as a molecular MRI contrast agent. The study reported an improved image quality, allowing improved detection of small positive lymph nodes on 3.0 T. There were fewer motion artifacts according to all 3 readers, and better lymph node border delineation according to 2 readers. Jensen et al. evaluated a 2D convolutional neural network based on the U-Net architecture for the zonal segmentation of the prostate gland on 1.5 T and 3.0 T MRI [25]. The study reported no significant difference between 3.0 T and 1.5 T MRI scanners in mean Dice similarity coefficients (0.778 ± 0.180 vs. 0.808 ± 0.102, respectively) or mean absolute distances (3.257 ± 1.665 mm vs. 3.431 ± 1.782 mm, respectively) of the central gland and no significant difference in Dice similarity coefficients (0.690 ± 0.148 vs. 0.694 ± 0.183, respectively) or mean absolute distances (3.000 ± 1.351 mm vs. 2.985 ± 1.59 mm, respectively) of the peripheral zones (p > 0.05).
Limitations
First, our meta-analysis was limited because of the small number of studies assessing 3.0 T and 1.5 T MRI (n = 4). Secondly, collaborating data from studies without standardized protocols or techniques (e.g. differences in endorectal coil use, phased-array coil use, and manufacturer factors) may result in bias with difficulty in interpretation and translation in clinical settings. Also, the studies have limited data on lesion dimensions, inter-reader variability, and flow and timing parameters. In addition, the location and size of the tumour were not analysed in the study. Finally, some of the included studies were done to assess image quality and endorectal coil usefulness rather than for initial staging, causing complexity in comparing with complete accuracy of both magnet strengths.
Conclusions
Our meta-analysis showed that the diagnostic performance of 3.0 T MRI was better than that of 1.5 T MRI for PCa, although without statistical significance. Despite the limitations of our study, our findings suggest the need for larger, prospective, randomized controlled trials directly comparing the use of 3.0 T and 1.5 T MRI in PCa.