Introduction
Diabetic peripheral neuropathy (DPN) is one of the most common complications of diabetes mellitus, and its prevalence has been estimated to be within the range 13–68% in patients with longstanding diseases [1]. DPN is closely correlated to neuropathic foot ulceration [2]. Furthermore, DPN is the leading cause of non-traumatic limb amputation [3]. Hence, if DPN can be diagnosed and treated early, the incidence of foot ulceration can be reduced.
Dyck [4, 5] proposed that abnormalities in two of five criteria (≥ 2 abnormalities from the neuropathy symptom score (NSS), neuropathy disability score (NDS), nerve conduction (NC), quantitative sensory examination (QSE), or quantitative autonomic examination (QAE), one of which is NC or QAE) are sufficient to diagnose DPN. The Toronto Diabetic Neuropathy Expert Group recommends that the diagnosis of DPN should be mainly based on symptoms, signs, and nerve conduction studies (NCS), and the measurement of small fibre neuropathy (SFN) [6].
NCS and the measurement of SFN are considered sensitive, objective, and reproducible approaches for assessing DPN. However, due to limited conditions (funding, laboratory, and invasiveness), the wide use of NCS and measurement of SFN has remained unrealistic. Therefore, the routine diagnosis of DPN has been based on clinical symptoms and signs, which are simpler approaches that can be used in an outpatient setting.
Clinically, the symptoms and signs of DPN can be quantified using validated scoring systems, such as NSS, NDS, [4, 5, 7] Toronto clinical scoring system (TCSS) [8, 9], and Michigan Neuropathy Screening Instrument (MNSI) [10].The evaluation of tendon reflexes is included in these clinical scores. In clinical practice, tendon reflexes have been used to evaluate DPN. Decreased or absent tendon reflexes are among the most common signs that suggest the possibility of DPN.
Hence, the aim of the present study was to evaluate the diagnostic efficacy of different tendon reflexes in detecting DPN.
Material and methods
From March 1, 2015, to December 31, 2017, patients with type-2 diabetes mellitus, who had regular follow-ups in the Diabetes Clinic of the Second Hospital of Hebei Medical University, were randomly selected for the present study. Two neurologists carefully evaluated each patient to exclude patients with other causes of DPN, such as alcohol abuse, exposure to neurotoxic drugs, endocrine, and inflammation. The present study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of The Second Hospital of Hebei Medical University. Written informed consent was obtained from all participants.
All patients were carefully interviewed and underwent neurological examinations, which included the assessment of conventional reflexes (biceps reflex, triceps reflex, radial periosteal reflex, patella reflex, and Achilles tendon reflex), mental status, cranial nerve function, sensory tests for a light touch, pin-prick, vibration and joint position sense, muscle strength, balance, gait, stretch reflexes, and coordination. The most common changes in tendon reflexes in diabetic patients were normal tendon reflexes, impaired (decreased or absent) Achilles tendon reflex only, impaired lower extremity tendon reflexes (Achilles tendon reflex and patella reflex), and impairment of all tendon reflexes (upper and lower extremity tendon reflexes).
According to the characteristics of the length-dependent DPN, the changes in tendon reflex can be divided into three strata. In stratum 1, the Achilles tendon reflex decreased or was absent, including the impaired Achilles tendon reflex only, impaired lower extremity tendon reflexes, and impairment of all tendon reflexes. In stratum 2, lower extremity tendon reflexes decreased or were absent, which included impaired lower extremity tendon reflexes and the impairment of all tendon reflexes. In stratum 3, all tendon reflexes decreased or were absent.
All patients were clinically assessed using the TCSS and MNSI. The TCSS was originally proposed by Perking [8, 9]. These patients were scored according to the score for symptoms (foot pain, numbness, tingling, weakness, imbalance, and upper limb symptoms), the score for tendon reflexes (bilateral patella and Achilles tendon reflexes), and the score for sensory signs (pin-prick, temperature, light touch, vibration, and position sense). Grading was stratified as follows: a score of 5 indicates no neuropathy, a score within 6–8 indicates mild neuropathy, a score within 9–11 indicates moderate neuropathy, and a score of 12 indicates severe neuropathy. The MNSI questionnaire was followed by a simple eight-point clinical examination, which involved the inspection of the foot, assessment of Achilles tendon reflexes, and semi-quantitative determination of vibration perception. An MNSI score of > 2 indicated the presence of neuropathy [10].
The nerve conduction velocity of all patients was measured via electromyography by skilled neurophysiologists. Standardised techniques for NCS with temperature control and fixed distances were applied. The measurement of latencies, distances, and amplitudes was performed in the standard fashion using onset latencies and baseline-to-peak amplitudes. For sensory curves, initial positive peak (if present) to negative peak measurements were used. The conduction velocities were automatically calculated by the Medtronic Keypoint EMG equipment. The F-wave program installed in the Medtronic Keypoint EMG equipment was used. F-waves were elicited by 20 supramaximal stimuli to measure the minimum and maximum latencies and calculate the mean latency for nerves. Then, the motor (median, ulnar, tibial, and peroneal) and sensory (median, ulnar, and sural) nerves were measured, and the data for compound muscle action potential (CMAP)/sensory nerve action potential (SNAP) and motor conduction velocity (MCV)/sensory conduction velocity (SCV) were obtained. Comprising stimulation of the median and ulnar nerves at the wrist (S1) and elbow (S2), and tibial and peroneal nerves at the ankle (S1) and knee (S2), the CMAP was recorded with the active electrode placed on the belly of the abductor pollicis brevis, abductor digiti minimi, abductor hallucis, and extensor digitorum brevis, and the reference electrode was placed 3 cm more distally. Another surface electrode pasted on the forearm or leg served as a ground. The following formula was used: MCV = distance (S2–S1)/latency (S2–S1). Stimulation of the median and ulnar nerves at the wrist (S), and sural nerves at the posterolateral calf (S). SNAP was recorded with the active electrode placed on the metacarpophalangeal joint of fingers I and V, Posterior malleolus (R). The following formula was used: SCV = distance (S – R)/latency R. According to NCS, the patients were divided into a normal NCS group and an abnormal NCS group. The tendon reflexes were divided into three groups: normal tendon reflexes, impaired Achilles tendon reflex only, and impaired lower extremity tendon reflexes (patella reflex and Achilles tendon reflex), to further study the relationship between tendon reflex and NCS.
Sensitivity and specificity
The sensitivity, specificity, and diagnostic accuracy of the tendon reflexes were calculated, with NCS as the gold standard. The true-positive, true-negative, false-positive, and false-negative findings were evaluated, and standard formulas were used to estimate the sensitivity, specificity, and diagnostic accuracy.
Statistical analysis
The results were analysed using the Statistical Package for Social Sciences (SPSS, Shanghai, China) version 17.0. Pearson’s χ2 test or independent samples t-test was used to examine the differences between different variables, as appropriate. The Shapiro-Wilk test was used to check the normal distribution of the examining variable before the t-test. In cases of non-normal distributions of the variable, the Mann-Whitney test was used. Skewed values were log-transformed before the analysis. A p-value of < 0.05 was considered statistically significant.
Results
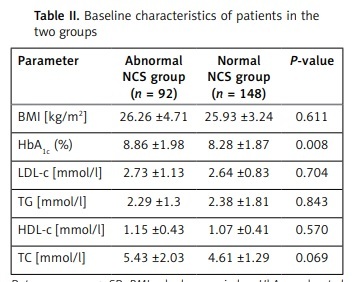
A total of 240 patients with diabetes were enrolled in this study. Among these patients, 123 (51.3%) were female, while 117 (48.7%) were male. The mean age of these patients was 56.5 ±12.4 years, and the disease duration of 110 patients was more than 10 years (45.8%). The mean value of glycated haemoglobin (HbA1c) was (8.26 ±1.67)%, while the value of the body mass index (BMI) was (26.06 ±3.87) kg/m2. Furthermore, abnormal NCS was observed in 92 (38.3%) patients, while normal NCS was observed in 148 (61.7%) patients. The impaired Achilles tendon reflexes were observed in 40 (16.7%) patients, impaired lower extremity tendon reflexes were observed in 76 (31.6%) patients, impairment of all tendon reflexes was observed in 37 (15.4%) patients, and normal tendon reflexes were observed in 87 (36.3%) patients. The mean values for TCSS and MNSI were 4.25 ±3.55 and 0.90 ±1.12, respectively. The clinical and demographic characteristics of the abnormal and normal NCS groups are presented in Table I. The TCSS scores revealed mild to severe neuropathy in 68.5% and 20.9% of patients in the abnormal NCS group and the normal NCS group, respectively (p < 0.001). Similarly, 47.8% of patients in the abnormal NCS group and 17.6% of patients in the normal NCS group had neuropathy, as determined by MNSI (p < 0.001). The metabolic parameters in the two groups are shown in Table II. A higher HbA1c level was observed in patients with abnormal NCS (p = 0.008).
Table I
Baseline characteristics of patients in the two groups
| Parameter | Abnormal NCS group (n = 92) | Normal NCS group (n = 148) | P-value |
|---|---|---|---|
| Age [years] | 58.6 ±10.94 | 55.21 ±13.10 | 0.035b |
| Gender: | |||
| Male | 42 | 75 | 0.449a |
| Female | 50 | 73 | |
| Duration of diabetes [years]: | |||
| 1–10 | 0 | 90 | 0.001a |
| > 10 | 52 | 58 | |
| TCSS: | |||
| < 6 | 29 | 117 | < 0.0001a |
| ≥ 6 | 63 | 31 | |
| MNSI: | |||
| ≤ 2 | 8 | 122 | < 0.0001a |
| > 2 | 44 | 26 | |
Table II
Baseline characteristics of patients in the two groups
The sensitivity and specificity of the reflexes and screening tests are shown in Table III. Taking NCS as the gold standard, stratum 1 yielded a sensitivity and specificity of 93.5% and 54.7%, respectively. Stratum 3 had higher specificity (96.6%), but lower sensitivity (34.8%), when compared to stratum 1. However, stratum 2 had the highest specificity (83.7% and 75.7% for sensitivity and specificity, respectively).
Table III
Sensitivity and specificity of tendon reflex and other screening tests with NCS as gold standard
The relationship between nerve conduction velocity and tendon reflexes was investigated (Table IV). The minimum latency of the F-wave in the group of abnormal lower extremities reflexes was longer when compared to that in the group of normal tendon reflex (61.3 ±5.6 vs. 45.0 ±4.3, p = 0.001) and the group of abnormal Achilles (61.3 ±5.6 vs. 57.0 ±6.3, p = 0.017). In addition, the latency F-wave in the group of abnormal Achilles was longer, when separately compared to that in the group of normal tendon reflex and the group of abnormal Achilles (45.0 ±4.3 vs. 57.0 ±6.3, p = 0.025). The SNAP and SCV of the sural nerve in the group of abnormal lower extremities reflexes was less than those in the group of normal tendon reflexes (2.0 ±0.8 vs. 4.5 ±2.3 and 38.5 ±4.7 vs. 56.7 ±5.9, p = 0.001 and p = 0.001, respectively) and the group of abnormal Achilles (2.0 ±0.8 vs. 2.5 ±1.2 and 38.5 ±4.7 vs. 45.1 ±5.3, p = 0.031 and p = 0.015, respectively). The SCV of the sural nerve in the group of abnormal Achilles was less than that in the group of normal tendon reflexes (2.5 ±1.2 vs. 4.5 ±2.3 and 45.1 ±5.3 vs. 56.7 ±5.9, p = 0.048 and p = 0.018, respectively). The CMAP and MCV of the tibial nerve in the group of abnormal lower extremities reflexes were less than that in the group of normal tendon reflexes (13.3 ±5.4 vs. 20.6 ±6.9 and 39.0 ±3.2 vs. 51 ±4.2, p = 0.001 and p = 0.001, respectively) and the group of abnormal Achilles (13.3 ±5.4 vs. 14.5 ±4.9 and 39.0 ±3.2 vs. 45.1 ±2.8, p = 0.012 and p = 0.016, respectively).
Table IV
Electrophysiological characteristics of diabetic patients with different tendon reflexes
Discussion
In the present study, we found that the changes in tendon reflexes were closely correlated to the incidence of DPN. Tendon reflexes could indicate DPN when there is a lack of NCS and measurement of the small fibre nerve. Furthermore, the Achilles tendon reflex and patella reflex display the most acceptable sensitivity, specificity, and predictive values. Thus, assessment of tendon reflexes could be proposed as a test for screening diabetic polyneuropathy.
The main purpose of screening for DPN is to treat it effectively in the early stages. At present, confirming DPN requires NCS or the measurement of SFN. However, the wide use of NCS and measurement of SFN remains limited because testing is time-consuming and costly, and there is limited availability of specialised laboratories. The efficacy of TCSS and MNSI scores has been validated for DPN in other studies [8–10]. However, these scores are too complex, time-consuming, and not suitable for screening DPN in diabetes or primary care clinics. As a result, physicians cannot routinely use these methods.
Impaired tendon reflexes are common in patients with DPN. In the present study, impaired tendon reflexes were found in 63.7% of patients with diabetes. Tendon reflex is a common and objective testing method, which can indirectly reflect the degree of injury of the peripheral nerve in neurological clinical practice. Decreased or absent deep tendon reflexes often indicate mild and asymptomatic DPN. An absent Achilles tendon reflex is considered the most sensitive index for screening DPN [11]. However, an absent Achilles tendon reflex has often been observed in healthy subjects, particularly in subjects aged > 70 years, because S1 nerve injury is induced by the atherosclerotic obstruction of the vasa vasorum or nutritional deficiency [12]. Because many factors influence the Achilles tendon reflex, the false-positive rate of the Achilles tendon reflex in the diagnosis of DPN is higher.
In clinical practice, it has been found that the absence of Achilles tendon reflexes is often accompanied by the absence of patella reflexes in subjects with diabetes. Age does not affect the patella reflex, and the intervertebral disc seldom squeezes the L2-L4 nerve root. Thus, the patella reflex may be more powerful than the Achilles tendon reflex when screening for DPN. However, it remains to be determined whether the diagnostic efficacy of an absent or decreased patella, or Achilles tendon reflexes, are higher than merely an absent or decreased Achilles tendon reflex.
In this study, we found that the sensitivity of stratum 1 was higher than that of stratum 2 (93.5% and 83.7%, respectively), while the specificity of stratum 2 was much higher than that of stratum 1 (75.7% and 54.7%, respectively). In addition, the diagnostic accuracy of stratum 2 was significantly higher than that of stratum 1 (78.8% and 69.6%, respectively). Thus, in the present study, the Achilles tendon reflex and patella reflex display the most acceptable sensitivity, specificity, and predictive values.
Based on the above findings, we conducted a further study to determine whether impaired lower extremity tendon reflexes were consistent with NCS. These tendon reflexes were divided into three groups: normal tendon reflexes, impaired Achilles tendon reflex only, and impaired lower extremity tendon reflexes. Our data showed that the changes in tendon reflexes were consistent with those in the neuroelectrophysiology group. The more weakened or absent the tendon reflex was, the more serious the impairment of the nerve conduction became. The F-wave is one of the most sensitive tools for diagnosing peripheral neuropathy [13], especially for DPN [14]. In the present study, when the ankle reflex decreased or was absent, the latency of the F-wave was prolonged. When the knee reflex decreased or was absent, the latency of the F-wave was further prolonged. Sural sensory nerves and tibial motor nerves are most susceptible to DPN. For DPN, nerve conduction velocity is more sensitive than amplitude. In the present study, when the ankle reflex was only impaired, the velocity of the sural nerve slowed down, but not the amplitude. Studies have shown that the sural nerve pathology in DPN was paranodal abnormalities of segmental demyelination and remyelination without axonal degeneration [15].
According to the above test results, incidences of DPN are closely correlated to changes in tendon reflexes. Tendon reflexes could indicate DPN when there is a lack of NCS and measurement of the small fibre nerve. If an Achilles tendon reflex is present in a diabetic patient, the possibility of DPN can be excluded. If all tendon reflexes are absent or diminished, DPN can probably be diagnosed. To our knowledge, our study is the first study to recommend the absence or decrease of Achilles tendon and patella reflexes as a screening tool for DPN, because this method can quickly and effectively distinguish DPN.
The present study has several limitations. First, it was a single-centre retrospective study, so selective bias cannot be avoided. Second, the number of patients was not adequate to draw an accurate conclusion. Furthermore, the sensory changes and the correlation with deep tendon reflexes were not evaluated in our patients. The correlation between tendon reflexes and the severity of peripheral nerve injury was also not evaluated.
In conclusion, our study demonstrated that the assessment of tendon reflexes could be proposed as a test for screening for diabetic polyneuropathy.