Introduction
Bladder cancer (BC) is one of the most common malignancies worldwide and non-muscle-invasive bladder cancer (NMIBC) accounts for 75% of the cases [1]. The NMIBC is a disease with a very high recurrence rate. Despite various additional procedures, including single dose intravesical chemotherapy and BCG immunotherapy, the recurrence rate after transurethral resection of bladder tumour (TURB) is still very high and a significant number of patients will develop muscle invasive disease [2]. It was shown that performing a restaging TURB (reTURB) allowed the disease to be properly staged and the recurrence and progression rates reduced. Additionally, reTURB identified residual tumours in 39% and 47% of patients primarily diagnosed with Ta and T1 tumours, respectively [3, 4]. Lately, Sfakianos et al. proposed that reTURB could have a beneficial impact on BCG immunotherapy outcome [5].
Despite clear guidelines of urological societies and common knowledge that a significant percentage of TURBs are not radical enough, in many cases a reTURB procedure is not performed by urologists [6, 7].
Aim
The aim of this study to analyse the influence of reTURB on outcomes in patients treated with BCG immunotherapy.
Material and methods
We analysed a database of 491 patients who were treated between 1998 and 2016.
Study inclusion criteria were tumours without lamina muscularis propria (LM) in the primary specimen and/or an intermediate and high risk (according to EORTC) Ta and/or any T1 urothelial tumours with or without concomitant carcinoma in situ (CIS). A re-staging resection was performed according to urologists’ decision based on pathologic and/or intraoperative outcomes. A minimum follow-up of 12 months was required. Patients with a diagnosis of muscle-invasive cancer, an interval between primary TURB and reTURB of more than 90 days, and those with a follow-up shorter than 12 months were excluded. Patients received immediate single instillation with a chemotherapeutic agent after primary TURB in accordance with EAU guidelines. ReTURB included resection of previous resection site and visible or suspected cancer foci.
Most of the specimens were originally evaluated by our team of three dedicated uropathologists. In the case of doubtful test results from other centres, specimens were reviewed and verified by our team. Tumour infiltration of lamina propria mucosae was described in the histopathological reports as no infiltration, focal infiltration or massive infiltration.
The whole BCG immunotherapy schedule consisted of 27 instillations divided into an induction course and seven maintenance courses. The induction course was composed of six weekly given instillations. Maintenance courses comprised three weekly given instillations administered after 3, 6, 12, 18, 24, 30 and 36 months [8]. Patients with at least 7 instillations (induction course and any maintenance) were included in the analysis.
A recurrence was defined as a recurrence of tumour of any stage and grade confirmed by TURBT and histologic or cytological assessment. Progression was defined as tumour relapse at tumour stage T2 or higher in the bladder or stromal invasion of the prostatic urethra.
Statistical analysis
The numerical results are presented as median with interquartile range (IQR). Group comparison was analysed with the Mann-Whitney test. The variations of frequencies were tested using the χ2 test. Times to events were calculated taking the date of initiating BCG as time zero. Recurrence-free survival (RFS), progression-free survival (PFS), cancer-specific survival (CSS) and overall survival (OS) were estimated using Kaplan-Meier curves. Patients without an event or death before an event were censored at the last date of follow-up. Differences in survival rates were assessed using a log-rank test. Times to events were also compared with the uni- or multivariate Cox proportional hazards regression model and hazard ratio (HR) with 95% confidence interval (95% CI). The value of adjusted p < 0.05 was considered statistically significant. All calculations were performed with Statistica v.13 (StatSoft, Poland).
Results
The study included 235 patients (age 65.7 ±8.7, 42 female/193 male) with a history of reTURB procedure and 256 patients (65.5 ±9.4 years, 49 female/207 male) without reTURB who were age and gender matched. In total 179 patients completed the whole BCG regimen (27 installations). The average number of BCG installations given was 19.36 in the reTURB group and 20.74 in the non-reTURB group.
The groups were not matched in terms of primary diagnosis (p < 0.001) (Table I), which reflects the reTURB qualification criteria. All the TaLG included in the analysis were in the intermediate risk group according to EORTC grading (e.g. because of size, multifocality, recurrent character), and therefore qualified for BCG. The inclusion criteria for reTURB in those cases included lack of lamina muscularis in the specimen or suspicion of residual disease.
Table I
Patients’ baseline characteristics according to reTURB status
The reTURB patients were followed from 12 to 163 months (median: 34, IQR: 22–62 months). Recurrence was observed in 64 (27%) with 10 months’ median time to the first recurrence (IQR: 3–21 months). Nineteen (8%) patients experienced recurrence more than once. Progression of the cancer was observed in 34 (14.5%) patients. The non-reTURB patients were followed from 12 to 257 months (median: 71, IQR: 35–118 months), with recurrences observed in 134 (52.3%), in 36 (14%) cases more than once. Median time to the first recurrence did not differ from the reTURB group and was 12 months, IQR 4–33 (p = 0.246). Progression was observed in 78 (30.5%) cases. Eventually, 74 (28.9%) patients underwent radical cystectomy in the non-reTURB group and 46 (19.5%) in the reTURB group. There were 16 (6.8%) and 50 (19.5%) cancer-specific deaths in reTURB and non-reTURB groups respectively. The patients were enrolled prospectively and the groups were not matched in terms of observation time, so we performed a survival analysis, avoiding direct comparison of frequencies of observational end-points.
Estimation of the frequency of recurrence and progression could only be performed including a subset of patients (189 with reTURB, 80 non-reTURB) who were included in the study after 2010. The subset presented a matched observation time between reTURB and non-reTURB groups (median: 31, IQR: 21–47 months vs. 33, IQR: 24–51 m, p = 0.208). There was a lower frequency of recurrences observed for reTURB patients (46, 24%) than non-reTURB (34, 43%, p = 0.003) and also a lower frequency of progression with 25 (13%) and 19 (24%, p = 0.033) cases, respectively. As the subset was not balanced in terms of qualification for the reTURB procedure, a detailed survival analysis was performed including the whole study population.
Recurrence-free survival
We observed at least one recurrence in 64 reTURB patients and 134 in the ones without reTURB. The RFS was significantly higher (p < 0.001) in the reTURB group (Figure 1).
Figure 1
Comparison of recurrence-free survival (Kaplan-Meier curves) between reTURB and without reTURB groups. Panels on the right side show distribution of the recurrences (only first recurrence) observed during the first 5 years of follow-up (white boxes show it quarterly over the first year)
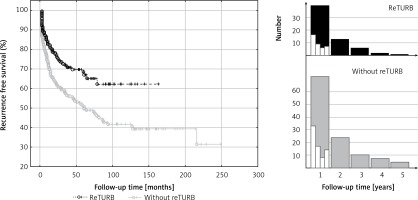
Most of the recurrences occurred shortly after BCG immunotherapy initiation (Figure 1). Out of 64 cases in the reTURB group, 39 (61%) occurred during the first year. In the non-reTURB group 72 out of 134 (54%) recurrences were observed during the first year. Residual tumour was present in 34% of patients with reTURB (T1HG 57 (30%) patients, T1LG 11 (52.4%) patients, TaHG 11 (35.5%) patients).
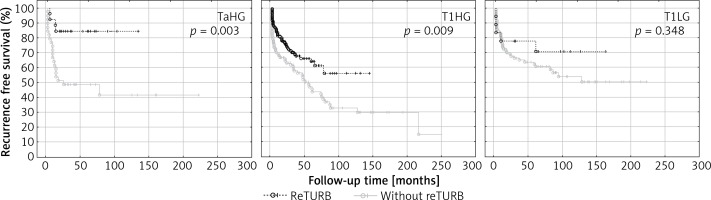
Table II and Figure 2 summarize the RFS observed in the setting of a particular cancer’s characteristics in relation to the reTURB procedure. The most pronounced improvement in RFS was related to TaHG and to cases without LM. The beneficial influence of reTURB is visible in most cases, not reaching statistical significance only for LG, T1LG (also with focal infiltration of lamina propria) and tumours with concomitant CIS.
Table II
Recurrence-free survival – reTURB vs. non-reTURB group comparison
[i] TURB – transurethral resection of bladder tumour, CIS – carcinoma in situ, HR – hazard ratio, 95% CI – 95% confidence interval, the value of adjusted p < 0.05 was considered statistically significant (bolded); HR > 1 better outcome when reTURB performed, HR < 1 worse outcome when reTURB performed, n.s. – non-significant.
Figure 2
Cancer type specific comparison of recurrence-free survival (Kaplan-Meier curves) between reTURB and without reTURB groups
When multivariate analysis of the tumour features influence on RFS in both study groups was performed, positive reTURB impact was confirmed and negative effect of massive infiltration of lamina propria was shown for patients without reTURB (Table III).
Table III
Recurrence free survival – clinical parameters’ influence in reTURB or without reTURB groups and multivariate analysis of factors
[i] F – female, M – male, TURB – transurethral resection of bladder tumour, CIS – carcinoma in situ, HR – hazard ratio, 95% CI – 95% confidence interval. The value of adjusted p < 0.05 was considered statistically significant (bolded); HR > 1 better outcome when reTURB performed, HR < 1 worse outcome when reTURB performed.
Progression-free survival
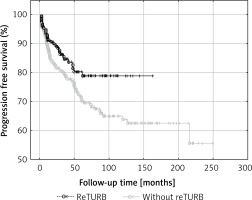
In the case of 34 (14.5%) reTURB patients and 78 (30.5%) non-reTURB ones cancer progression was observed. PFS was significantly higher (log-rank p = 0.031) in the reTURB group (Figure 3).
Figure 3
Comparison of progression-free survival (Kaplan-Meier curves) between reTURB and without reTURB groups (p = 0.031)
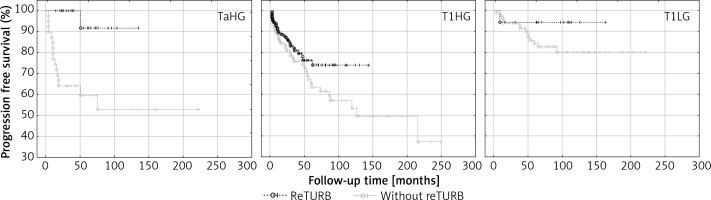
An improvement in PFS due to reTURB was observed for TaHG tumours (Table IV and Figure 4).
Table IV
Progression free survival – reTURB vs. non-reTURB group comparison
[i] TURB – transurethral resection of bladder tumour, CIS – carcinoma in situ, HR – hazard ratio, 95% CI – 95% confidence interval, the value of adjusted p < 0.05 was considered statistically significant (bolded); HR > 1 better outcome when reTURB performed, HR < 1 worse outcome when reTURB performed.
Figure 4
Cancer type specific comparison of progression-free survival (Kaplan-Meier curves) between reTURB and without reTURB groups (TaHG log-rank p = 0.001, Cox HR = 12.4)
In the multivariate analysis a positive influence of reTURB on PFS was confirmed. A negative influence of HG and concomitant CIS on PFS was observed in non-reTURB cases (Table V) and was excluded by the reTURB procedure.
Table V
Progression free survival – clinical parameters’ influence in reTURB or non-reTURB groups
[i] F – female, M – male, TURB – transurethral resection of bladder tumour, CIS – carcinoma in situ, HR – hazard ratio, 95% CI – 95% confidence interval, the value of adjusted p < 0.05 was considered statistically significant (bolded), HR > 1 better outcome when reTURB performed, HR < 1 worse outcome when reTURB performed.
Overall survival
Thirty-eight reTURB and 102 patients without reTURB died during the observation. Cancer-specific death occurred in 16 cases of reTURB patients and 50 non-reTURB ones. Data presented in Table VI show that both OS and CSS were significantly improved by the reTURB procedure in HG and T1HG tumours.
Table VI
Overall survival – reTURB vs. non-reTURB group comparison
[i] TURB – transurethral resection of bladder tumour, CIS – carcinoma in situ, OS – overall survival, CSS – cancer-specific survival, HR – hazard ratio; 95% CI – 95% confidence interval, the value of adjusted p < 0.05 was considered statistically significant (bolded), HR > 1 better outcome when reTURB performed, HR < 1 worse outcome when reTURB performed.
In multivariate analysis a borderline (p = 0.097) positive influence of reTURB on CSS was noted. A negative influence of HG on OS and CSS was observed in non-reTURB cases (Table VII) and was excluded by the reTURB procedure. On the other hand, a similar beneficial impact was not observed in the case of massive infiltration, which negatively influenced OS and CSS even in reTURB patients.
Table VII
Overall and cancer–specific survival – clinical parameters’ influence in reTURB or non-reTURB groups
[i] F – female, M – male, OS – overall survival, CSS – cancer-specific survival, TURB – transurethral resection of bladder tumour, CIS – carcinoma in situ, HR – hazard ratio, 95% CI – 95% confidence interval, the value of adjusted p < 0.05 was considered statistically significant (bolded); HR > 1 better outcome when reTURB performed, HR < 1 worse outcome when reTURB performed.
Discussion
In the available literature, despite abundant reports concerning the reTURB procedure, there is a scarcity of papers analysing reTURB in the BCG-treated population, especially in patients receiving both induction and maintenance courses.
Recurrences
Available reports state that recurrences after single TURB can reach up to 70% of cases depending on tumour stage or grade [2, 9–11]. It was previously shown that reTURB reduces the recurrence risk especially in short-term follow-up [3, 12, 13].
In our study, we were able to prove that reTURB reduces the risk of tumour recurrence for both general and subgroups analysis. However, despite the lower risk of a recurrence in the reTURB group, the median times to the first recurrence were not statistically different between the two groups.
When the tumour subgroups were analysed, a statistically significant improvement in RFS was observed for T1HG and TaHG tumours in the reTURB group compared to non-reTURB cases. Patients with T1LG tumours were not shown to clearly benefit from reTURB, however, the subgroup of T1LG was small and probably not representative (Figure 2). It should be emphasized that our results concerning TaHG tumours are inconsistent with current (2018) EAU NMIBC guidelines. When estimating the frequency of recurrence, it was proved that for all tumours a lower frequency of recurrences was observed for reTURB patients than for non-reTURB ones. Concomitant CIS did not influence the RFS in any of the groups.
Various authors state that TURB which is not deep enough to contain the detrusor muscle in the histopathological specimen is associated with worse treatment results. Lack of LM may be a reason for staging inaccuracies and is considered an independent predictor of progression to muscle-invasive disease [14, 15]. In our study, one-fifth of patients, for whom the information about presence of LM in the histopathological sample was available, did not have a detrusor muscle in the TURB specimen. In the case of those patients, there was a much greater improvement in RFS due to the reTURB procedure than for LM-positive cases.
The highest rate of recurrences (more than 25%) was observed during the first 3 months. In the non-reTURB group, patients had double the risk of experiencing a recurrence compared to the reTURB group. However, this is most likely due to persistence of disease rather than recurrence [2]. Some authors state that even up to 80% of patients have persistent disease at reTURB and 30% of persistent tumours are muscle invasive [5, 12, 16]. In this study residual tumour was present in 34% of patients including 35.5% for TaHG. Those findings are consistent with other studies, however, the real number may be slightly different due to the fact that patients with T2 disease on reTURB were not qualified for BCG and were lost to further observation.
The influence of BCG immunotherapy on T1 cancers depending on the different depths of invasion has rarely been addressed previously [17–19]. In the mentioned series, the authors presented conflicting results regarding tumour recurrence and progression rates. However, the analysed groups were small and different classification systems were used. In this study, analysis of the level of lamina propria infiltration in T1 cancers showed that massive invasion was associated with shorter RFS compared to focal infiltration in non-reTURB cases. It was also shown that reTURB performed in patients with massive infiltration have a positive and statistically significant influence on RFS (Figure 5). When the influence of particular histopathological tumour features on RFS was analysed within the study groups, the degree of lamina propria infiltration in T1 tumours was found to be significantly associated with RFS in the group without reTURB and the negative prognosis related to massive infiltration was diminished by reTURB.
Figure 5
Comparison of recurrence-free survival (Kaplan-Meier curves) according to lamina muscularis propria infiltration and reTURB performance. There was a statistically significant difference in RFS between massive and focal infiltration in patients without reTURB (log-rank p = 0.027, focal inf. HR = 0.49, 95% CI: 0.30–0.81) and between reTURB yes/no for massive infiltration (log-rank p = 0.027, no reTURB HR = 2.09, 95% CI: 1.10–3.96)
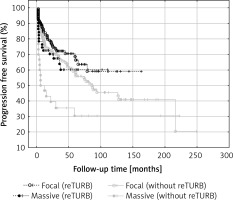
Progression
Progression rates of NMIBC can reach up to 40% especially when concomitant CIS is present [20]. Some authors state that performing reTURB allows to lower the risk of progression [3, 12, 13]. In the analysis of this study population it was shown that reTURB decreases the risk of cancer progression for both general and tumour subgroup analysis. Despite the lower risk of the median times to progression were not statistically different between the two groups.
When tumour subgroups were analysed, the most pronounced benefit from the reTURB procedure was observed for TaHG tumours. Once more, this result is contradictory with current EAU NMIBC guidelines. In cases of T1HG or T1LG, there was also a similar but less pronounced borderline effect. The reason might be the relatively short follow-up time in some reTURB cases. In multivariate general analysis a positive influence of reTURB on PFS was confirmed. When estimating the frequency of observed progression, it was found that for all tumours there was a lower frequency of progression for reTURB patients than for non-reTURB cases.
It was previously proposed that prognosis for T1 cancers may differ depending on the level of lamina propria infiltration [19, 21]. In the literature, progression rates vary from 55% for T1c cancers to 10% in T1a [19, 22, 23]. In this study, analysis of the level of lamina propria infiltration in T1 tumors demonstrated that massive invasion was correlated with shorter PFS. In the case of reTURB patients the presence of focal invasion was related to lower progression risk. Despite the clear of this study results, it should be taken into account that the relative number of endpoints in progression analysis was limited.
Deaths
General OS was not proved to be reTURB dependent. When analysing general CSS for both uni- and multivariate analysis a borderline association was noted. However, when the Kaplan-Meier curves were analysed, the benefits from reTURB could be observed in a longer period of time (Figure 6). What is more, collected data showed that both OS and CSS were significantly improved by the reTURB procedure in T1HG tumours and in all HG ones. When analysing the influence of LM presence in specimen on CSS it was revealed that there were no statistically significant differences. Similarly, there was no influence of reTURB on CSS in patients with or without LM. However, these results may be influenced by the low number of endpoints. On the other hand, analysis of lamina propria infiltration of T1 tumours revealed that massive infiltration is associated with worse CSS compared to the focal group.
Figure 6
Comparison of cancer-specific survival (Kaplan-Meier curves) between reTURB and without reTURB groups
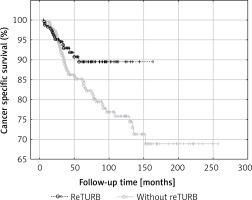
Our results match the outcomes obtained by Gontero et al. in large, recent analysis of T1HG patients treated with BCG. They stated that reTURB impacted favourably recurrence, progression and survival rates, only when there was absence of muscle in histopathological specimen at primary TURB [24]. Our results also are coherent with results of other analyses of similar populations stating that a single TURB was a strong predictor for disease recurrence and progression [5].
However, our results are in contrast with outcomes obtained by Holmang [25]. He stated that a routine second resection is probably not necessary among TaG3 patients based on the observation that uncertain benefits may be lower than procedure cost and morbidity. Yet, it has been proved in other studies that patients with TaHG tumours have a lifelong risk of disease stage progression and death from BC similar to those with T1 tumours [26]. In our opinion, Holmang’s observation may be true only in the situation of certainty that the operation was complete and histopathological examination was high-quality.
Bearing in mind the previous reports, the presented results and the risk of tumour underdiagnosis, we believe that T1 cancers with both focal and massive lamina propria infiltration should undergo reTURB [27, 28]. Additionally, because of poor prognosis rates and safety profile of adjuvant procedures, radical treatment should be considered, especially when massive infiltration was found [29–32].
Limitations and strengths
Firstly, the long period of the observation raises doubts about whether a cohort effect may occur with present-day high-risk patients being dissimilar from those from early part of the study. However, during 20 years of the course, none of the qualification, therapeutic and maintenance details changed.
Secondly, although our department is a referral department in uro-oncology and is the only centre offering BCG immunotherapy in the region, the population of our patients underwent resections in various centres. For that reason, TURBs and histopathological assessment may have been performed with different quality. However, the majority of the specimens were originally evaluated or were re-evaluated by our team of pathologists to avoid incorrect classification.
Thirdly, there is a lack of information about the proportion of patients diagnosed with T2 cancers at primary and reTURB. Those patients were disqualified from BCG immunotherapy, and therefore lost to observation.
Despite its limitations, our study has some clear strengths. Firstly, we present a large and homogeneous Centro-European population which was not previously included in BCG trials. Secondly, our group has a long and meticulous follow-up with minimal loss to observation. Thirdly, our patients received maintenance courses in “6 + 3” manner, which was rarely thoroughly studied in previous reports.
Conclusions
This study highlights the importance of reTURB in patients with NMIBC before initiating BCG immunotherapy. In our study, it was demonstrated that reTURB allowed for improvement of recurrence-free, progression-free and cancer-specific survival. In this population, patients with TaHG tumours benefited the most. It was also demonstrated that massive lamina propria infiltration in T1 tumours is associated with the worst recurrence-free, progression-free and cancer-specific survival.









