Introduction
Vascular reconstruction is one of the methods frequently used for the treatment of obstructive arterial diseases; however, the success of these interventions is less than expected because of development of thrombosis or stenosis [1]. Unlike the formation of acute thrombus that causes sudden obstruction after reconstructive vascular interventions, neointimal hyperplasia due to smooth muscle cell migration and proliferation as well as extracellular matrix accumulation plays an important role in stenosis and restenosis in the late period [2]. In the animal and human models of arterial injury, smooth muscle cell proliferation and connective tissue accumulation in intima have been defined as the main causes of lumen stenosis [3].
Each arterial reconstruction procedure causes partial endothelial injury. The most common cause of this injury is the various degrees of trauma occurring during the graft removal procedure and anastomosis. Subendothelial fibroproliferation and formation of neointima are the intimal responses to endothelial injury. Although this intimal neoplastic response is a part of vascular repair after trauma, it might be unnecessarily severe under certain conditions. As a result of excessive neointimal proliferation, impairment of the anticoagulant property of endothelium, and lumen stenosis, blood flow decreases and thrombosis may occur in some cases [4]. More et al. observed unusual intimal thickening after 3 days of ballooning in rabbits, re-endothelialization started, and on the 14th day this process was completed. It has been determined that intimal thickening reaches the maximum level at the end of the first month and decreases within 3 months due to extracellular matrix accumulation [5].
Vascular inflammation developed by free oxygen radicals resulting from leukocyte and thrombocyte activation in the coronary angioplasty region plays a major role in vascular restenosis [6]. Antioxidants may modify neointima formation and vascular remodeling by inhibiting this inflammatory reaction [7]. Glutathione acts as a nucleophilic scavenger and an antioxidative catalyst in oxidative tissue injury [8]. Thus, it has a major role in preserving biological structures and functions. Intracellular reduced glutathione plays an important role in the protection of endothelial cells against free oxygen radicals [9]. It has been demonstrated to enhance the endothelial vasomotor response to acetylcholine when directly infused into the coronary arteries [10]. Intravenous N-acetylcysteine (NAC) administration has also been demonstrated to have similar effects as glutathione on endothelial functions due to its capability of inducing glutathione synthesis [11]. Studies conducted by Jeremias et al. have shown that NAC administered at the dose of 150 mg/kg/day effectively increases serum glutathione levels [12].
N-acetylcysteine is the N-acetylated derivative of the natural amino acid L-cysteine. N-acetylcysteine displays a direct antioxidant property by interacting with the electrophilic group of oxidative radicals via its free thiol (-SH) nucleophilic group [13]. N-acetylcysteine, which easily enters into the cell owing to its molecular structure, is acetylated there and turns into L-cysteine. L-cysteine is a glutathione precursor and enhances glutathione synthesis [14–16]. Glutathione is a highly reactive tripeptide, which protects cells against harmful effects of exogenous or endogenous cytotoxic substances and oxidative radicals, and plays a major role in an endocellular mechanism for the maintenance of cellular integrity and functions [17]. From this aspect, NAC is of primary importance in glutathione synthesis at a level that would protect the cells against injury. N-acetylcysteine is a source of sulfhydryl groups. Sulfhydryl groups have many biological functions, including scavenging free oxygen radicals and modifying the half-life of NO [18].
Various clinical trials and animal studies, in which various agents including lipid-lowering drugs, anti-platelet agents, immunosuppressants, and antioxidants were investigated to reduce neointimal hyperplasia and to prevent lumen stenosis, have been conducted [19].
In the present study, the effect of NAC, which is an antioxidant, on intimal hyperplasia and endothelial proliferation after carotid anastomosis was investigated in a rabbit model. For this purpose, histological findings of anastomosed and opposite non-anastomosed carotid arteries of the same experimental animals were compared in two distinct experimental groups, which either received NAC or did not.
Material and methods
Experimental animals
The present randomized, controlled, experimental trial study was started after the approval of Dokuz Eylül University Medical Faculty, Experimental Animals Ethics Committee. The study included 14 randomly selected New Zealand-type male rabbits weighing 2–3 kg on average. In the course of the study, all experimental animals were kept under the same conditions (in a room at temperature of 20 ±2°C with a ventilation system and receiving sunlight) and fed rabbit feed. Rabbits were divided into two groups. The control group (n = 7) underwent right carotid artery anastomosis and received no medication. The NAC group (n = 7) underwent right carotid artery anastomosis and received NAC for 21 days following surgery. NAC was administered at a dose of 150 mg/kg/day just after the surgery. The initial dose was given via the intravenous route and subsequent doses were given via the intramuscular route.
Anastomosis
All anastomoses were performed by the same researcher. A cannula was preoperatively inserted into the marginal vein of the ear of the rabbits. Anesthesia was induced with 50 mg/kg intramuscular ketamine and 5 mg/kg intramuscular xylazine. Incision areas were shaved to provide better vision during surgery and disinfected with povidone-iodine. The right carotid artery was explored through a right vertical neck incision. Intravenous heparinization was performed at a dose of 100 IU/kg. The right carotid artery was transected after clamping proximal and distal segments with a bulldog clamp. Afterwards, anastomosis was completed by suturing one by one with 8-0 polypropylene suture, and tissues were closed in an anatomical layer. The procedure was performed using a loop with 3.5× magnification.
All experimental animals were alive in the course of the study. None of the rabbits developed any wound site infection or neurological problem at the end of the postoperative 28th day. All experimental animals were sacrificed using pentobarbital at the end of the 28th day, and the right anastomosed and the left non-anastomosed carotid artery segments were removed and sent to the histology laboratory for examination.
Histopathological examination
After right and left carotid artery segments were fixed with 10% buffered formaldehyde solution and embedded in paraffin, 5 µm serial sections were obtained from these paraffin blocks using a rotary microtome (Leica RM 2135, Leica Instruments, Nussloch, Germany). These sections were stained with hematoxylin and eosin and additionally with Verhoeff-Van Gieson stain. The prepared slides were examined under the light microscope (Olympus BX-50, Tokyo, Japan). Furthermore, the acquired images were transferred to a computer via a high-resolution camera (Olympus DP-70, Japan) and then evaluated by a digital image analysis program (Image Tool, UTHSC Image software for Windows 3.0, Texas University, USA). During the examination of vascular tissue, tunica intima and tunica media thicknesses, tunica intima and tunica media areas, vascular diameters, and vascular lumen areas were measured with serological methods. These parameters were assessed for both anastomosed and non-anastomosed arteries of the control and NAC groups.
Statistical analysis
Data were analyzed using SPSS (SPSS Inc., Chicago, IL, USA) version 15.0. Data were expressed as mean ± standard deviation. For non-normally distributed numerical variables, comparison between independent two groups was performed using the Mann Whitney-U test and comparison between two dependent groups was performed using the Wilcoxon signed-rank test. A p-value < 0.05 was considered significant.
Results
The results of the histological measurements of anastomosed and non-anastomosed carotid arteries of the NAC and control groups are presented in Table I. The images of internal elastic lamina of the groups stained with hematoxylin and eosin are shown in Figure 1, and Verhoeff elastic stain images are shown in Figure 2.
Table I
Histological measurements of the anastomosed and non-anastomosed carotid arteries of the study groups
Figure 1
Images of internal elastic lamina of the groups stained with hematoxylin and eosin (A – control group, non-anastomosed, B – control group, anastomosed, C – NAC group, anastomosed. IEL – internal elastic lamina)
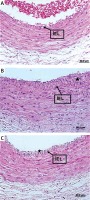
Figure 2
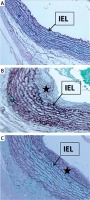
Verhoeff elastic stain images of the groups. A – Verhoeff elastic stain of the non-anastomosed arteries in the control group. B – Verhoeff elastic stain of the carotid anastomosis in the control group, the elastic fibrils were disorganized and irregular. C – Verhoeff elastic stains of the carotid anastomosis in the N-acetylcysteine administered group, the elastic fibrils were more organized and regular compared to those in the control group
→ indicates membrane elastic interna, *intimal hyperplasia.
The mean values of lumen, intima and media areas and the ratio of intima area/media area of non-anastomosed arteries were similar in the control and NAC groups, whereas they were significantly different for anastomosed arteries except media area. Lumen diameter of both the anastomosed and non-anastomosed arteries showed a significant difference between the control and NAC groups; it was also significantly narrower in the anastomosed arteries compared to that in the non-anastomosed arteries in both groups (Table I). While lumen area was significantly lower in the anastomosed arteries of the control group, no difference was observed between anastomosed and non-anastomosed arteries of the NAC group. Carotid artery intima area was found to be significantly larger in the anastomosed arteries than that in the non-anastomosed arteries in both the control and NAC groups. While media area was significantly larger in the anastomosed arteries of the control group, no difference was found between anastomosed and non-anastomosed arteries of the NAC group. A significantly higher intima/media ratio was observed in the anastomosed arteries as compared to non-anastomosed arteries in both groups (Table I).
Comparing the percentages of changes in the parameters of both the control and NAC groups after anastomosis, the reduction in the lumen diameter and area was significantly smaller in the NAC group compared to the control group. Although the percentage increases in intima and media areas were lower in the NAC group, there was no significant difference between the control and NAC groups (Table II).
Table II
Percentage of changes in the parameters after anastomosis in the study groups
Discussion
N-acetylcysteine is considered to have important cytoprotective effects [8]. Endogenous antioxidants (such as glutathione) are depleted during tissue reperfusion following sepsis, trauma, burns, pancreatitis, hepatic failure, hemorrhage, and acute myocardial infarction and related cellular injuries and thus they may mediate production and release of numerous free radicals. N-acetylcysteine is an oxidative free radical scavenger. Moreover, NAC is a glutathione precursor through its capability of replacing depleted intracellular glutathione and its role in the additional antioxidant defense theory [20, 21]. In studies, NAC has been demonstrated to be successful in preventing nephropathy in experimental models [22, 23]. N-acetylcysteine stimulates glutathione synthesis and potentiates nitroglycerine-mediated coronary artery vasodilatation as well [24]. Likewise, it potentiates the inhibitory effect of platelet aggregation of nitroglycerine [25]. A study on an animal model demonstrated that NAC reduced the release of matrix metalloproteinases from macrophages, called foam cells, in atherosclerotic plaques, and thus it contributed to plaque stabilization [26].
Recent studies have shown that NAC is an important regulator of nuclear factor-κB (NF-κB) activity in endothelial and smooth muscle cells. NAC regulates neointimal formation by promoting upregulation of specific adhesion molecules and post-injury vascular cell activation of endothelial cells and smooth muscle cells via NF-κB inhibition [27].
In their study, Ghigliotti et al. [28] compared NAC and heparin in a rabbit model of abdominal aortic balloon injury and reported that NAC provided considerable reduction in smooth muscle cell proliferation and intimal hyperplasia and substantially contributed to vascular remodeling. In a study by Jeremias et al. [29], although a significant increase in glutathione levels was obtained due to NAC administration after experimental endothelial damage in the iliac arteries of rabbits via balloon angioplasty, no significant difference was observed regarding restenosis and inflammatory markers between the groups. In contrast, Mass et al. [30] reported significant decreases in inflammatory markers, endothelial damage, thrombus formation, and elastic lamina injury in the rabbit carotid arteries in an experimental balloon injury model after NAC administration. They suggested that NAC had the potential of inhibiting the inflammatory process developed after mechanical vascular damage. In these three studies, the damage caused by the balloon is only in the intima layer. We believe that the anastomosis model used in our study is a more suitable model for intimal hyperplasia developed after surgery because it damages all the layers of the artery.
In the present study, while reduction in the lumen area and diameter after anastomosis was significantly improved in the NAC group as compared to the control group, increases in the intima and media areas and intima/media ratio were smaller in the NAC group after anastomosis as compared to those in the control group, but the difference was not significant. We concluded that this effect of NAC resulted from its antioxidant property and its capability of regulating proliferation and migration of vascular smooth muscle cells [31, 32]. In a study by Kanber et al. [33], early restenosis in diabetic and non-diabetic patients undergoing carotid endarterectomy was evaluated but no difference was found. More information may be provided as a result of the examination of the vessel patency in similar patient groups using NAC.
In conclusion, we opine that vascular anastomosis and post-intervention NAC administration will prolong vascular patency by reducing intimal hyperplasia and providing vascular remodeling.


