Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
HEMATOLOGY / CLINICAL RESEARCH
Effectiveness of bendamustine in relapsed or refractory lymphoma cases: a Turkish Oncology Group study
1
Department of Medical Oncology, Health Sciences University, Gulhane Training
and Research Hospital, Ankara, Turkey
2
Department of Medical Oncology, Faculty of Medicine, Cukurova University, Adana,
Turkey
3
Department of Medical Oncology, Health Sciences University, Dr. Abdurrahman
Yurtaslan Training and Research Hospital, Ankara, Turkey
4
Department of Medical Oncology, Medicalpark Hospital, Ankara, Turkey
5
Department of Medical Oncology, Health Sciences University, Numune Training
and Research Hospital, Ankara, Turkey
6
Department of Medical Oncology, Faculty of Medicine, Baskent University, Adana,
Turkey
7
Department of Medical Oncology, Faculty of Medicine, Hacettepe University, Ankara,
Turkey
8
Department of Haematology, Faculty of Medicine, Cukurova University, Adana,
Turkey
9
Department of Haematology, Faculty of Medicine, Gazi University, Ankara, Turkey
10
Department of Haematology, Faculty of Medicine, Koc University, Istanbul, Turkey
11
Department of Haematology, Health Sciences University, Gulhane Training
and Research Hospital, Ankara, Turkey
12
Department of Medical Oncology, Special Tinaztepe Hospital, Izmir, Turkey
13
Department of Medical Oncology, Anatolian Health Center, Istanbul, Turkey
Submission date: 2018-02-21
Final revision date: 2018-06-17
Acceptance date: 2018-08-07
Online publication date: 2019-02-18
Publication date: 2021-07-16
Arch Med Sci 2021;17(4):920-927
KEYWORDS
TOPICS
ABSTRACT
Introduction:
We aimed to investigate the efficacy and side effects of bendamustine in relapsed/refractory lymphoma patients in Turkey.
Material and methods:
In this retrospective study, we included relapsed/refractory Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) patients who underwent multiple lines of chemotherapy. The primary endpoint was to determine the objective response and toxicity.
Results:
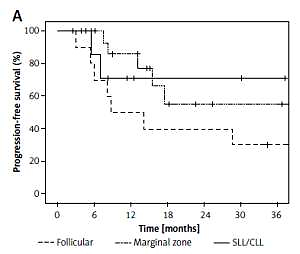
Ninety-nine patients with a median age of 59.8 years were included in the study. Eighty-one patients had NHL (follicular lymphoma: 10, diffuse large B-cell lymphoma: 27, mantle-cell lymphoma: 18, marginal zone lymphoma: 9, small lymphocytic lymphoma/chronic lymphocytic leukemia: 17) and 18 patients had HL. The patients had previously received a median of three lines of chemotherapy (range: 2–8) except autologous stem cell transplantation (ASCT); 19 patients (HL: 11, NHL: 8) had undergone ASCT. The objective response rate (ORR) was 74.3%, the complete response rate was 57% (= 53), and the partial response rate was 16.6% ( = 19). The overall survival (OS) rate at 1 year was 74.6%. The progression-free survival (PFS) rate at 1 year was 62.5%. The most common side effects were lymphopenia, anemia and neutropenia. Side effects which were observed as grade 3 and higher levels were lymphopenia (14.1%), neutropenia (10.1%) and fatigue (7.1%).
Conclusions:
Objective response rate of bendamustine was found to be 74.3% in relapsed/refractory HL and NHL patients. It appears to be an effective option as a salvage treatment for patients who have previously received multiple lines of therapy.
We aimed to investigate the efficacy and side effects of bendamustine in relapsed/refractory lymphoma patients in Turkey.
Material and methods:
In this retrospective study, we included relapsed/refractory Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) patients who underwent multiple lines of chemotherapy. The primary endpoint was to determine the objective response and toxicity.
Results:
Ninety-nine patients with a median age of 59.8 years were included in the study. Eighty-one patients had NHL (follicular lymphoma: 10, diffuse large B-cell lymphoma: 27, mantle-cell lymphoma: 18, marginal zone lymphoma: 9, small lymphocytic lymphoma/chronic lymphocytic leukemia: 17) and 18 patients had HL. The patients had previously received a median of three lines of chemotherapy (range: 2–8) except autologous stem cell transplantation (ASCT); 19 patients (HL: 11, NHL: 8) had undergone ASCT. The objective response rate (ORR) was 74.3%, the complete response rate was 57% (= 53), and the partial response rate was 16.6% ( = 19). The overall survival (OS) rate at 1 year was 74.6%. The progression-free survival (PFS) rate at 1 year was 62.5%. The most common side effects were lymphopenia, anemia and neutropenia. Side effects which were observed as grade 3 and higher levels were lymphopenia (14.1%), neutropenia (10.1%) and fatigue (7.1%).
Conclusions:
Objective response rate of bendamustine was found to be 74.3% in relapsed/refractory HL and NHL patients. It appears to be an effective option as a salvage treatment for patients who have previously received multiple lines of therapy.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.