Introduction
Inguinal hernia refers to the protrusion of the abdominal cavity contents to the body surface through a defect of the abdominal wall in the inguinal region, which can be cured by standard surgical therapy, and inguinal hernia in adults should be treated surgically. According to the literature [1, 2], the incidence rate of inguinal hernia is about 0.1–0.5%. Currently, laparoscopic herniorrhaphy is a commonly adopted method of herniorrhaphy in clinical practice, which has the advantages of less trauma, a clear field of vision, high safety and reliability in tissue separation and mesh placement, and remarkable efficacy. However, all surgery leads to trauma and will cause a stress response, inducing varying degrees of damage to the immune function [3, 4]. Different anesthetic methods lead to different stress response intensities and immune function damage [5, 6]. At present, the influences of different anesthetic methods on the immune function and oxidative stress in patients are studied mostly in tumorectomy, whereas such influences in laparoscopic herniorrhaphy are relatively less investigated. Herniorrhaphy is more common in clinical practice.
Aim
Therefore, we herein evaluated the influences of different anesthetic methods on the immune function and oxidative stress in patients undergoing laparoscopic herniorrhaphy, aiming to find out a more suitable anesthetic method for maximizing the efficacy of surgical therapy.
Material and methods
General information
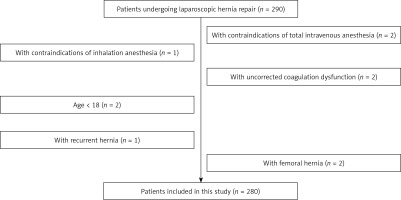
A total of 280 patients undergoing laparoscopic herniorrhaphy in our hospital between January 2018 and November 2020 were prospectively selected (Figure 1). Inclusion criteria: (1) Patients meeting the diagnostic criteria in Guidelines for diagnosis and treatment on the adult groin hernia (2018 edition) [7], and (2) those definitely diagnosed with direct or indirect inguinal hernia based on medical history inquiry and auxiliary examinations. Exclusion criteria: (1) Patients with contraindications for total intravenous anesthesia (TIVA) or inhalation anesthesia (IA), (2) those with uncorrected coagulation dysfunction, (3) underage patients, (4) those with recurrent hernia, (5) those with femoral hernia, or (6) those with other surgery-intolerable contraindications. The 280 patients were divided into the TIVA group (n = 140) and the IA group (n = 140) using the random number table method. In the TIVA group, there were 76 males and 64 females, aged 39–71 years old, with a mean of 59.2 ±6.3 years old. In the IA group, there were 72 males and 68 females, aged 41–73 years old, with a mean of 59.4 ±5.9 years old. No significant differences were observed in the clinical baseline data between the two groups (p > 0.05) (Table I). The present study was approved by the Ethics Committee of our hospital, and all patients and their family members signed the informed consent.
Table I
Baseline clinical data
Anesthesia methods
At 30 min before the operation, patients in both groups were intramuscularly injected with 0.5 mg of atropine (NMPN: H32020166, Jiangsu Lianshui Pharmaceutical Co., Ltd.) and 5 mg of diazepam (NMPN: H41020631, Tianjin Pharma Jiaozuo Co., Ltd.). Anesthesia was induced by intravenous injection of 4 μg/kg fentanyl (NMPN: H42022076, Yichang Humanwell Pharmaceutical Co., Ltd.) and 0.1 mg/kg midazolam, followed by 2 mg/kg propofol (NMPN: H20010368, Xi’an Libang Pharmaceutical Co., Ltd.). When the patients lost consciousness, they were intravenously injected with 0.15 mg/kg cisatracurium besilate (NMPN: H20060869, Shanghai Hengrui Pharmaceutical Co., Ltd.). After the patients’ muscles were relaxed, endotracheal intubation was conducted. During the operation, cisatracurium besilate was intravenously injected every 30 min (Dosage: 10 mg of drug was dissolved in 5 ml of sterile water for injection. The dose was adjusted according to the patients’ neuromuscular function) to maintain muscular relaxation, and then mechanical ventilation was carried out. Anesthesia was induced in both groups using the same method. In the IA group, anesthesia was maintained by inhalation of isoflurane (NMPN: X19990127, Abbott Laboratories Limited) with end-tidal concentration of 1%, which was stopped 30 min before the end of the operation. In the TIVA group, anesthesia was maintained by continuous intravenous infusion of remifentanil (NMPN: H42022076, Yichang Humanwell Pharmaceutical Co., Ltd.) and propofol (NMPN: H20010368, Xi’an Libang Pharmaceutical Co., Ltd.) using a micro-pump. Propofol was maintained at a dose of 4 mg/(kg·h) until 10 min before the end of the operation, and remifentanil was given at 0.1 μg/(kg·min) until 5 min before the end of the operation. In this study, the operation was performed by members of the same operation team, and the anesthesia depth monitor (G9L multi-parameter anesthesia depth monitor, Danmeter ApS, Denmark) was used to monitor anesthesia throughout the operation.
Observation of anesthetic effects
The operation time, anesthesia duration and extubation time were recorded in both groups.
Measurement of immune cell levels
Before anesthesia, at the end of the operation, and during 7:00-8:00 am at 1 and 3 days after the operation, 5 ml of venous blood was collected from each patient, and the immune-related indices, including T lymphocyte subsets cluster of differentiation 3+ (CD3+), CD4+ and CD8+, and the level of CD4+/CD8+ were detected in both groups using a Coulter Epics XL flow cytometry (USA).
Measurement of levels of oxidative stress response indices
Before anesthesia, at the end of the operation, and during 7:00–8:00 am at 1 and 3 days after the operation, 5 ml of venous blood was collected from each patient, and the levels of catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) were measured by the thiobarbituric acid method.
Measurement of levels of inflammatory factors
Before anesthesia, at the end of the operation, and during 7:00–8:00 am at 1 and 3 days after the operation, 5 ml of venous blood was collected from each patient, and the levels of immune factors were measured. Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were determined by enzyme-linked immunosorbent assay (ELISA) according to the instructions of the ELISA kits (Shanghai Jining Shiye Co., Ltd.), and the level of C-reactive protein (CRP) was determined by immunoturbidimetry.
Observation of adverse reactions
The incidence of adverse reactions, including irritability, nausea and excessive sedation, was compared between the two groups.
Statistical analysis
SPSS 21.0 software was used for statistical analysis. The measurement data in line with normal distribution were expressed as mean ± standard deviation (χ ± s), and the t-test was used for comparison between groups. The numerical data were expressed as percentage, and the χ2 test was employed for comparison between groups. P < 0.05 suggested that a difference was statistically significant.
Results
Anesthetic effects
There were no significant differences in the operation time, anesthesia duration and extubation time between the two groups (p > 0.05) (Table II).
Levels of immune cells
At the end of the operation and at 1 day after the operation, CD3+ and CD4+ T cells and CD4+/CD8+ were decreased in both groups in contrast with those before anesthesia (p < 0.05). At 3 days after the operation, levels of CD3+ and CD4+ T cells were also lower than those before anesthesia (p < 0.05), and they were significantly higher in the TIVA group than those in the IA group (p < 0.05). However, there was no significant difference in the level of CD8+ T cells between the two groups at each detection time (p > 0.05) (Table III).
Table III
Levels of immune cells at each time point
| Index | Group | N | Before anesthesia | At end of operation | 1 day after operation | 3 days after operation |
|---|---|---|---|---|---|---|
| CD3+ | TIVA | 140 | 58.55 ±5.46 (51–63) | 52.13 ±4.89# (47–56) | 54.41 ±4.76# (50–58) | 60.21 ±5.68# (54–67) |
| IA | 140 | 58.89 ±5.51 (53–64) | 48.87 ±4.92# (44–53) | 52.56 ±4.82# (48–57) | 54.29 ±5.74*,# (51–60) | |
| CD4+ | TIVA | 140 | 32.36 ±3.23 (29–36) | 26.57 ±2.45# (24–30) | 28.73 ±3.24# (27–32) | 35.11 ±3.45# (32–38) |
| IA | 140 | 32.86 ±3.42 (29–36) | 25.13 ±2.51# (23–28) | 27.66 ±3.19# (24–31) | 28.82 ±3.28*,# (26–32) | |
| CD8+ | TIVA | 140 | 25.68 ±2.45 (23–28) | 24.79 ±2.52 (22–26) | 24.89 ±2.62 (23–27) | 24.22 ±2.46 (23–27) |
| IA | 140 | 25.76 ±2.43 (23–28) | 24.61 ±2.37 (23–28) | 25.19 ±2.43 (23–28) | 24.78 ±2.56 (22–28) | |
| CD4+/CD8+ | TIVA | 140 | 1.32 ±0.18 (1.11–1.52) | 1.06 ±0.09# (0.92–1.15) | 1.13 ±0.11# (0.90–1.24) | 1.32 ±0.13 (1.16–1.45) |
| IA | 140 | 1.30 ±0.20 (1.08–1.48) | 0.98 ±0.08# (0.89–1.06) | 1.08 ±0.12# (0.95–1.17) | 1.24 ±0.11 (1.12–1.32) |
Levels of oxidative stress response indices
At the end of the operation and 1 and 3 days after the operation, the oxidative stress indices SOD, CAT and GSH-Px declined in both groups in comparison with those before anesthesia (p < 0.05), and they were significantly higher in the IA group than those in the TIVA group at each time point (p < 0.05) (Table IV).
Table IV
Levels of oxidative stress response indices
| Index | Group | N | Before anesthesia | At end of operation | 1 day after operation | 3 days after operation |
|---|---|---|---|---|---|---|
| SOD [U/ml] | TIVA | 140 | 107.26 ±11.27 (89.25–120.18) | 78.76 ±5.27# (73.24–84.13) | 86.42 ±5.11# (82.15–91.24) | 98.22 ±5.74# (94.37–103.22) |
| IA | 140 | 106.92 ±11.32 (94.27–118.24) | 60.28 ±5.33*,# (58.23–64.92) | 72.38 ±4.81*,# (68.25–76.29) | 85.39 ±5.25*,# (84.27–90.17) | |
| CAT [U/ml] | TIVA | 140 | 80.34 ±7.21 (73.22–87.73) | 71.56 ±4.41# (67.25–75.49) | 74.73 ±3.22# (71.22–77.43) | 77.12 ±3.45# (74.26–80.18) |
| IA | 140 | 80.45 ±7.33 (73.25–87.19) | 63.14 ±4.51*,# (59.25–67.288) | 65.62 ±3.14*,# (64.29–68.56) | 69.82 ±3.22*,# (66.25–73.41) | |
| GSH-Px [U/ml] | TIVA | 140 | 196.34 ±15.43 (174.23–215.19) | 154.72 ±12.45# (135.27–167.25) | 174.82 ±12.78# (159.28–186.45) | 182.21 ±12.45# (175.43–193.24) |
| IA | 140 | 197.04 ±16.38 (184.23–214.38) | 134.62 ±12.43*,# (123.29–148.24) | 155.11 ±12.49*,# (143.27–167.32) | 164.71 ±12.52*,# (158.28–178.34) |
Levels of inflammatory factors
At the end of the operation and at 1 and 3 days after the operation, there was no significant difference in the level of TNF-α between the two groups (p > 0.05). However, the levels of IL-6 and CRP were significantly elevated in contrast with those before anesthesia (p < 0.05). Moreover, they were lower in the TIVA group than those in the IA group at the end of the operation and at 1 and 3 days after the operation (p < 0.05) (Table V).
Table V
Levels of inflammatory factors
| Index | Group | N | Before anesthesia | At end of operation | 1 day after operation | 3 days after operation |
|---|---|---|---|---|---|---|
| IL-6 [pg/ml] | TIVA | 140 | 58.97 ±5.46 (54.38–63.27) | 66.89 ±5.22# (61.27–72.34) | 64.39 ±4.12# (60.28–68.29) | 60.21 ±3.56# (57.28–63.42) |
| IA | 140 | 58.29 ±5.31 (53.29–64.02) | 79.22 ±5.32*,# (74.25–84.32) | 69.02 ±4.23*,# (65.29–73.22) | 64.32 ±3.54*,# (62.45–67.92) | |
| TNF-α [ng/ml] | TIVA | 140 | 33.31 ±2.22 (30.22–35.46) | 31.52 ±2.35 (30.34–33.42) | 31.54 ±2.32 (29.87–33.46) | 29.97 ±2.41 (28.98–33.15) |
| IA | 140 | 33.42 ±2.32 (29.32–35.42) | 32.16 ±2.44 (29.48–34.87) | 31.61 ±2.24 (29.68–33.57) | 29.81 ±2.33 (28.76–32.41) | |
| CRP [μg/ml] | TIVA | 140 | 71.31 ±5.41 (67.89–76.34) | 80.43 ±4.89# (76.43–84.38) | 75.84 ±4.71# (71.46–78.94) | 74.21 ±4.43# (70.39–78.19) |
| IA | 140 | 71.08 ±5.36 (66.87–76.18) | 89.62 ±4.45*,# (84.27–93.25) | 83.12 ±4.45*,# (78.28–87.43) | 80.72 ±4.53*,# (77.34–85.02) |
Adverse reactions
The incidence rate of adverse reactions was significantly higher in the IA group than that in the TIVA group (25.00% vs. 11.43%) (χ2 = 9.447, p = 0.002) (Table VI). Probably, IA easily stimulated the respiratory tract, so the incidence rates of irritability and nausea were high.
Discussion
Surgical trauma brings continuous and varying stimulation to the body, and the acceptance of the traumatic stress response varies in patients [8]. After trauma, the oxidative stress response will be enhanced in the body, and an excessive stress response will aggravate the injury, influencing the immune function to a certain extent [9, 10]. The perioperative stress response is mainly caused by anesthesia or surgical trauma [11, 12]. Since the surgical method for the patient is determined, choosing an appropriate anesthetic method is of great importance to reduce the perioperative stress response and immune injury in the body. Therefore, in this study, patients undergoing laparoscopic herniorrhaphy in our hospital were prospectively selected, and the influences of different anesthetic methods on the immune function of patients were analyzed. Moreover, changes in immune function of patients were judged by detecting the levels of immune cells and inflammatory factors.
T lymphocytes are the major cells involved in cellular immunity. CD3+ T cells are the total T cells, and CD4+ T cells can promote T lymphocyte maturation and stimulate B lymphocytes to produce antibodies, while CD8+ T cells mainly kill target cells. Hence, the levels of these cells can represent the immune state of the body to a certain extent, and determination of these cells can reflect the immune function of patients [13]. In this study, by detecting the levels of T lymphocyte subsets, it was found that CD3+ and CD4+ T cells and CD4+/CD8+ cells were all significantly decreased at the end of the operation and at 1 day after the operation in contrast with those before anesthesia (p < 0.05). Moreover, at 3 days after the operation, CD3+ and CD4+ T cells were also significantly reduced compared with those before anesthesia (p < 0.05). However, the level of CD8+ T cells at each detection time was not significantly different from that before anesthesia in both groups (p > 0.05). Also, the levels of CD3+ and CD4+ T cells were significantly higher in the IA group than those in the TIVA group at 3 days after the operation (p < 0.05). These observations suggest that anesthesia can indeed cause immune injury, and the mechanism may be as follows: On the one hand, anesthesia has a certain impact on the synthesis, differentiation and transformation of DNAs of lymphocytes, which decreases the levels of CD3+ and CD4+ T cells and causes an imbalance in CD4+/CD8+, resulting in the destruction of the immune barrier and the decline of immune function. On the other hand, anesthesia will also inhibit immune-related cell channels and enzyme systems, thus suppressing immune function. Meanwhile, it can be seen that TIVA has relatively little impact on the immune level of patients, probably because most inhaled anesthetics, such as enflurane and nitrous oxide, inhibit immune cells [14, 15]. The antioxidant function and myocardial protective function of propofol are also beneficial for the maintenance of immune function.
The antioxidant system can be divided into enzymatic antioxidation and non-enzymatic antioxidation, and SOD, CAT and GSH-Px in the enzymatic antioxidant system are components of the body’s physiological protection system [16]. Under external stimulation, the body will secrete more highly active molecules such as reactive nitrogen free radicals and reactive oxygen free radicals, resulting in an imbalance between antioxidant and oxidation systems, and thereby inducing tissue injury in the body. Overexpression of SOD can reduce the number of dead neurons in the hippocampal CAI region when patients have focal ischemia or global cerebral ischemia [17]. In addition, SOD can protect cortical embolism and blood-brain barrier injury after oxidative stress. The results of this study revealed that the oxidative stress indices SOD, CAT and GSH-Px declined in both groups at the end of the operation and at 1 and 3 days after the operation in contrast with those before anesthesia (p < 0.05), and they were significantly higher in the IA group than those in the TIVA group at each time point (p < 0.05). These results suggest that TIVA can effectively reduce the oxidative stress response in patients after the operation, thus decreasing the tissue injury caused by oxidative stress response.
CRP is an index to reflect the acute phase reaction after trauma, while IL-6 is a major factor that induces hepatocytes to synthesize CRP. The serum levels of IL-6 and CRP after the operation are positively correlated with the degree of stimulation to the patients after the operation, so monitoring the dynamic changes in them can help evaluate the recovery of patients after the operation. The results of this study revealed that at the end of the operation and at 1 and 3 days after the operation, there was no obvious change in the level of TNF-α, but the levels of IL-6 and CRP were significantly elevated in both groups compared with those before anesthesia (p < 0.05). Moreover, there were significant differences in the levels of IL-6 and CRP between the IA group and the TIVA group at the end of the operation and at 1 and 3 days after the operation (p < 0.05). These results suggest that macrophages and lymphocytes are activated in both groups after the operation. It has previously been reported that TIVA was superior to IA, probably because the protective effect of propofol on immune function suppresses the inflammatory response caused by surgical stimulation [18]. In addition, the adverse reactions were also compared between the two groups in this study. The incidence rate of adverse reactions in the IA group was significantly higher than that in the TIVA group (25.00% vs. 11.43%). It can be seen that under the same anesthetic effect, TIVA has less impact on the immune function of patients, and the incidence rate of adverse reactions is lower.
Nevertheless, this study has some limitations. First, the dose of anesthetics was not taken into account, and indices were detected in a limited time range. Second, early complications that affected the body’s systemic response were not recorded. Further in-depth studies are ongoing in our group.