Introduction
Cigarette smoke contains approximately 4700 chemical compounds, some of which are toxic, mutagenic and carcinogenic. Smoking is known to have negative effects on human health. So far, it has been shown to lead to lung cancer, chronic bronchitis, and coronary heart diseases, while its adverse effects on reproductive issues appear to be less known [1]. However, smoking leads to sudden infant death syndrome, premature births, decreased fertility in women, early menopause and diminished ovarian reserve [2]. Although the prevalence of smoking has decreased globally, the number of smokers remained constant from 2007 to 2015 (1.1 billion in number) [3]. It has been reported that the prevalence of cigarette smoking, especially in women of reproductive age, is increasing worldwide, and one third of women in this age group are smokers [4].
Females are born with a fixed number of ovarian follicles in their ovaries, and this certain number of ovarian follicles, in which the development of the primordial follicles is arrested in meiosis stage I, are gathered together as primary, secondary, antral follicles in order to ovulate in puberty [4]. This follicular growth process continues until follicular pool depletion and menopause. Depletion of ovarian reserve, secondary to genetic abnormalities, aggressive treatments (radiotherapy of cancer), ovarian surgery (for endometriosis), idiopathic and the negative effects of various environmental and lifestyle factors such as smoking may go through acceleration periods [5].
In smokers, follicular survival and cellular viability have been found to decrease in relation to increased pro-apoptotic markers, namely Bax, activated caspase 3, HSP 90ab1 and DNA fragmentation [6]. As a result of exposure of newborn ovaries to smoking, apoptosis was induced, and caspase 2–3 levels and DNA fragmentation were increased as well [7]. The chemicals that act on the aryl hydrocarbon receptors (AhR) in the cigarette have been shown to induce apoptotic pathways [8]. The depletion of the primordial follicle (PMF) pool due to apoptosis and subsequent follicular atresia appears to be one of the possible mechanisms of premature ovarian failure (POF) and infertility [9].
Reactive oxygen species (ROS) have been suggested to accumulate in the environment during the follicular growth in the long period of arrest of meiosis stage I. ROS-related mitochondrial DNA damage and mutations, telomere shortening and dysfunction are associated with reduced number and quality of oocytes [10]. ROS have been shown to be related to apoptotic cell death in ovarian follicles and granulosa cells and diminished antioxidant defense reactions [5]. The imbalance between the pro-oxidant and antioxidants in the follicular environment of the ovaries has been found to be associated with impaired folliculogenesis [11]. Present data pointed out that oxidative stress induces telomere dysfunction and ends with chromosomal instability and apoptosis [12].
Cigarettes contain many chemical-reactive molecules (such as carbon dioxide, hydrogen peroxide, nitrogen oxides) that contain reactive oxygen derivatives and free radicals. A significant increase in oxidative stress-induced apoptosis in rat ovarian tissues cultured with dimethylbenzanthracene (DMBA) (a chemical in cigarettes) was apparent whereas culture with glutathione demonstrated that cell death was prevented by antioxidants in natural conditions [4].
Melatonin (N-acetyl-5-methoxytryptamine) is a neurohormone produced in very different tissues such as the brain, eye, immune system and reproductive system [13, 14]. Melatonin is known to be a powerful antioxidant molecule that acts through direct and indirect mechanisms [15]. Melatonin and its metabolites exert their direct activities through the process of cleaning ROS and reactive nitrogen species (RNS) [16, 17]. Melatonin is found to be produced in ovaries, cumulus cells, oocytes, follicular fluid and placenta in terms of reproductive function [14].
In this experimental study, we aimed to investigate the protective effects of melatonin, an antioxidant molecule, on oxidant-antioxidant balance and apoptotic pathways with respect to the negative impact of smoking on the ovarian reserve in cigarette smoke exposed rats.
Material and methods
Animal maintenance and treatment
The ethical approval was licenced by Kocaeli University Animal Research Ethics Committee (Project number 2015/37, Kocaeli, Turkey). The principles of the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978) were followed. All experiments were carried out between 9:00 a.m. and 12:00 p.m. under standard laboratory conditions (22±2°C room temperature, 12-h light/dark cycle with lights on at 7:00 a.m.). Tap water and food pellets were provided equally.
Seventy-two female Wistar-Albino rats were divided into 6 groups: group I (control group to be exposed to room air, n = 12), group II (smoke exposure group, n = 12), group III (room air + 10 mg/kg melatonin group, n = 12), group IV (room air + 20 mg/kg melatonin group, n = 12), group V (smoke exposure group + 10 mg/kg melatonin group, n = 12), group VI (smoke exposure group + 20 mg/kg melatonin group, n = 12).
In groups exposed to smoking, applying the ISO3308:2012 (Routine analytical cigarette smoking machine – definitions and standard conditions) standard and using the exposure device designed in accordance with the standard and the FTC (Federal Trade Commission) method, 2 times a day for 1 h each, a total of 10 cigarettes per day were used. Each cigarette contained 9.7 mg tar, 0.85 mg nicotine and 11.7 mg TPM (total particulate matter per cubic meter of air). A total of 45 days of exposure was ensured. Each smoking exposure cycle in the machine consisted of 1 cigarette puff per 2 s per cycle, followed by a 28-second draw time, a total of 30 s. The cigarette puff cycle was restarted after following the 30-second fresh air puff for each smoking cycle. In the groups where room air was planned to be given, room air was supplied with the same machine. Melatonin administration was performed as subcutaneous injection at doses of 10 mg/kg and 20 mg/kg, 2 h before the lights were turned off once a day. Melatonin injection was performed in the last 15 days of 45-day exposure [17–19].
Tissue sample collection
At the end of the experiment, anesthesia was performed on all of the rats by intraperitoneal injection of ketamine (12.5 mg/kg) and xylazine (2.5 mg/kg) and blood samples were acquired by cardiac puncture. Blood samples were immediately centrifuged and stored at –40°C for consequent analysis. After the collection of blood samples, all the rats underwent oophorectomy by an appropriate surgical procedure. Left ovaries were fixed in 4% paraformaldehyde solution for light and fluorescein microscopic tissue processing. Right ovaries were quickly frozen at –40°C for biochemical analysis.
Light and fluorescein microscopic examinations
The left ovarian tissues were dehydrated through ascending grades of ethanol and cleared in toluene, then embedded in paraffin. Serial sections of 5 µm thickness were cut from each ovary and applied to poly-l-lysine coated microscope slides. Sections of the ovaries were stained with hematoxylin and eosin (H&E) to determine follicle count and ovarian structure. Caspase-3 and TUNEL-fluorescein methods were applied to the sections derived from the central portion of ovaries for immunohistochemical analysis.
Histological analysis and follicle count
H&E staining was applied on every 10th section of the ovary so that each section was separated by a distance of approximately 50–60 µm from the next 10th section to evaluate the ovarian structure and follicle count [20]. Ovarian follicles were counted and classified according to the following follicle type: primordial follicle, primary follicle, secondary follicle, Graafian (mature) follicle [21]. All sections were evaluated with an optical microscope (Olympus CX41, Tokyo, Japan) and monitored with cellSens digital imaging software (Ver. 1.7.1) by an attached digital camera (Olympus DP26, Tokyo, Japan).
Anti-caspase-3 immunohistochemical analysis
Sections cut from the central portion of the ovary from each group were chosen to determine anti-caspase-3 immunoreactivity. 5-µm-thick sections of the ovaries were de-paraffinized in toluene (2 × 10 min), rehydrated through descending grades of ethanol (100, 96, 90, and 70%; 5 min each) and rinsed twice for 2 min each with distilled water. The slides were placed for 5 min in an antigen retrieval solution, 0.01 M citrate buffer (pH = 6.0) in a microwave oven. The endogenous peroxidase was inhibited with 0.3% H2O2 (Merck, Darmstadt, Germany) in methanol for 10 min. Then, the slides were rinsed twice for 5 min each with phosphate-buffered saline (PBS, pH = 7.6) containing 0.3% Triton X-100 (PBS/Triton). The tissue sections were covered for 10 min by a protein block solution (Mouse and rabbit specific HRP/DAB (ABC) detection IHC kit, Abcam, ab64264, UK) for blocking non-specific binding. Then the slides were rinsed once for 5 min in PBS/Triton. The tissue sections were covered with rabbit polyclonal anti-caspase-3 (ab4051; Abcam, UK) primary antibody (dilution factor = 1 : 500) and then incubated overnight at 4°C in a humidified chamber [22]. After rinsing the tissue sections with PBS/Triton (4 × 5 min), biotinylated secondary antibody (Mouse and rabbit specific HRP/DAB (ABC) detection IHC kit, Abcam, ab64264, UK) was applied for 10 min at a room temperature. Then, slides were washed with PBS/Triton (4 × 5 min), streptavidin peroxidase solution was applied (Mouse and rabbit specific HRP/DAB (ABC) detection IHC kit, Abcam, ab64264, UK), and slides were incubated for 10 min. Afterward, the tissue sections were rinsed with PBS/Triton (4 × 5 min) and 3,3'-diamino benzidine (DAB) solution (Mouse and rabbit specific HRP/DAB (ABC) detection IHC kit, Abcam, ab64264, UK) was performed as a chromogen for 10 min. Slides were washed with PBS/Triton (4 × 5 min), adequate drops of Mayer’s hematoxylin were added to cover the sections for counterstaining and they were incubated for 1 min. The tissue sections were rinsed 7–8 times in tap water and mounted with mounting medium (ab64230; Abcam, UK) for light microscopic examination. All sections were evaluated with an optical microscope (Olympus CX41, Tokyo, Japan) and monitored with cellSens digital imaging software (Ver. 1.7.1) by an attached digital camera (Olympus DP26, Tokyo, Japan). Anti-caspase-3 expression intensity of follicular cells was determined by a histological score (HSCORE) method. The intensity of anti-caspase-3 immunostaining was semiquantitatively evaluated by the following categories: 0, no staining; 1+, weak staining; 2+, moderate or intermediate staining; 3+, intense or strong staining [23]. For each tissue section, the HSCORE value was created by summing the percentage of cells that were stained at each intensity category and multiplying that value by the weighted intensity of the staining, using the formula HSCORE = ∑Pi (i + 1), where i is the intensity score, and Pi is the percentage of labeled cells for each intensity within a range of 0–100%.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay
In situ cell death detection was assessed by fluorescent labeling with the TUNEL method as described by the manufacturer (1168479591; Roche Applied Science, Indianapolis, IN, USA). In brief, 5-µm-thick sections of the ovaries were de-paraffinized in toluene (2 × 5 min), rehydrated through descending grades of ethanol (100, 95, 70, and 30%; 5 min each) and rinsed once for 3 min with distilled water. Excess water was removed on slides with a Kimwipe and tissue sections were surrounded by PAP pen. Then, sections were incubated for 15 min at 37ºC in a solution of proteinase-K (pH = 7.4, ab64220; Abcam, UK) diluted to 20 µg/ml in PBS for membrane permeabilization. Slides were rinsed twice for 3 min in PBS. Thereafter sections were incubated with the provided fluorescein-conjugated TUNEL reaction mixture in a humidified chamber for 60 min at 37°C in the dark. After incubation, slides were rinsed 3 times for 3 min in PBS. The sections were then mounted with mounting medium (ab64230; Abcam, UK). TUNEL-stained nuclei were examined under an inverted fluorescence microscope (Olympus IX53, Tokyo, Japan) (excitation wavelength: 515–565 nm).
Tissue homogenization and serum preparation
The ovarian tissues were weighed and homogenized in a 1/10 (weight /volume) ratio of phosphate-buffered saline (PBS; 0.1 M, pH 7.4) with a tissue homogenizer. The homogenates were centrifuged at 5000 rcf for 5 min to separate the supernatants and stored in Eppendorf tubes at –40°C until the time of analysis.
In order to obtain serum samples, blood samples taken into the tube without anticoagulant were centrifuged at 3500 rpm for 10 min and the supernatants were stored at –40 C until analysis.
Tissue protein determination
Total protein determination was performed by a modified Lowry method. The tissue results of the parameters to be analyzed were determined by proportioning to the amount of tissue protein.
Determination of tissue and serum MDA levels and CAT, GPx, SOD activities
MDA, GPx, SOD and CAT measurements were conducted in tissue and serum by commercial enzyme-linked immunosorbent assay (ELISA) kits (E-EL-0060, E-EL-R2456; Elabsience Biotechnology Co., Wuhan, China, 201-11-1705, 201-11-0169; Sunred Biotechnology Co., Shanghai, China, respectively) according to the manufacturer’s instructions.
Statistical analysis
The statistical analysis was carried out using IBM SPSS 20.0 (SPSS Inc., Chicago, IL, USA). The Shapiro-Wilk test was used to assess the normality of the data. Comparisons of multiple samples were performed using one-way ANOVA with post-hoc Tukey test for parametric variables with normal distributions and data were expressed as the mean±standard deviation (SD). The Kruskal-Wallis test was performed for nonparametric variables and data were reported using the median and interquartile range (IQR: 25–75th percentile). Tests were performed within the 95% confidence interval and the significance level was determined to be based on a p-value <0.05.
Results
A total of 72 female Wistar-Albino rats were enrolled in the study. In group II and group III, 2 rats died (one in each group) and therefore were excluded from the study. With 12 rats in group I, IV, V, VI each and 11 rats in group II and III each, the study was completed with 70 rats in total.
Biochemical parameters
Biochemical data are shown in Table I.
Table I
Mean ± SD of tissue and serum SOD, tissue and serum GPx, tissue and serum CAT, tissue and serum MDA levels in control, smoke exposure, room air + 10 mg melatonin, room air + 20 mg melatonin, smoke exposure + 10 mg melatonin and smoke exposure + 20 mg melatonin groups
| Parameter | Control | Smoke exposure | Room air + 10 mg melatonin | Room air + 20 mg melatonin | Smoke exposure + 10 mg melatonin | Smoke exposure + 20 mg melatonin |
|---|---|---|---|---|---|---|
| Tissue SOD [U/mg protein] | 14.97 ±3.08 | 8.72 ±3.62* | 15.27 ±4.28+ | 17.37 ±5.65+ | 9.96 ±2.33* | 14.15 ±4.36+ |
| Tissue GPx [ng/mg protein] | 64.09 ±19.76 | 32.42 ±13.08* | 67.69 ±26.73+ | 101.52 ±29.55*,+ | 44.60 ±21.58 | 62.30 ±20.64+ |
| Tissue CAT [U/mg protein] | 128.15 ±17.99 | 85.84 ±17.14* | 144.91 ±19.19+ | 161.25 ±19.52*, + | 102.69 ±27.20 | 116.48 ±25.46+ |
| Tissue MDA [ng/mg protein] | 90.79 ±31.79 | 186.68 ±24.68* | 86.79 ±27.13+ | 77.88 ±17.29+ | 132.55 ±35.20*+ | 108.90 ±20.76+ |
| Serum SOD [U/ml] | 8.69 ±2.57 | 4.03 ±0.91* | 10.71 ±1.82+ | 12.27 ±2.27*,+ | 6.05 ±1.32* | 7.52 ±1.96+ |
| Serum GPx [ng/ml] | 17.25 ±5.24 | 10.96 ±3.51* | 16.57 ±2.97+ | 29.51 ±4.70*,+ | 15.37 ±6.29+ | 18.56 ±5.87+ |
| Serum CAT [U/ml] | 50.13 ±6.56 | 31.45 ±8.76* | 59.23 ±15.96+ | 61.96 ±11.45+ | 42.33 ±13.18 | 47.93 ±12.06+ |
| Serum MDA [ng/ml] | 50.17 ±11.01 | 84.88 ±18.92* | 52.98 ±11.43+ | 42.28 ±8.95+ | 65.36 ±12.11*+ | 55.60 ±8.66+ |
Tissue and serum MDA levels
Serum and tissue levels of MDA were significantly higher in the smoke exposure group in comparison with the control group (p < 0.05). Significantly reduced MDA levels were detected in the treatment groups in comparison with the smoke exposure group (p < 0.05). No significant difference was determined between treatment groups (p > 0.05).
Tissue and serum GPx, SOD, CAT activities
Tissue and serum GPx, SOD, CAT activities were found to be significantly reduced in the smoke exposure group compared to the control group (p < 0.05). Those parameters were shown to be significantly increased in all treatment groups, especially in smoke exposure + 20 mg/kg melatonin group (p < 0.05) in comparison with the smoke exposure group. No significant difference was determined between treatment groups (p > 0.05).
Light microscopy
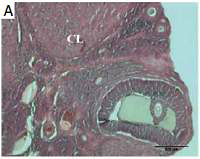
H&E staining demonstrated that ovarian tissues of the control group (group I) exhibited normal histological structure whereas cystic and degenerated follicles were observed in the smoking group (group II). Degenerated follicles were observed in the smoking + 10 mg/kg melatonin group (group III) while the histological structure of ovarian tissues was similar to the control group in the smoking + 20 mg/kg melatonin group (group IV). General ovarian structure, stroma and follicle images in all groups are presented in Figure 1. The follicle counts are shown in Table II.
Table II
Number of ovarian follicles in control, smoke exposure, room air + 10 mg/kg melatonin, room air + 20 mg/kg melatonin, smoke exposure + 10 mg/kg melatonin and smoke exposure + 20 mg/kg melatonin groups
| Parameter | Control | Smoke exposure | Room air + 10 mg melatonin | Room air + 20 mg melatonin | Smoke exposure + 10 mg melatonin | Smoke exposure + 20 mg melatonin |
|---|---|---|---|---|---|---|
| Primordial follicle count | 11 ±3.59 | 4 ±1.73* | 9.18 ±2.14+ | 10.67 ±2.23+ | 7.25 ±1.82*,+ | 9.83 ±3.21+ |
| Primary follicle count | 9.75 ±3.25 | 4.09 ±1.3* | 6.18 ±2.09* | 8.17 ±2.62+ | 5.67 ±2.53* | 7.08 ±1.88+ |
| Secondary follicle count | 6 ±2.52 | 2.55 ±1.37* | 4.45 ±1.69 | 5.33 ±1.87+ | 3.75 ±2.45 | 4.92 ±1.16+ |
| Graafian (mature) follicle count | 2.5 (2.0–3.75) | 1.0 (0.0–2.0)* | 1.0 (0.0–3.0)* | 2.0 (1.0–3.0) | 1.5 (0.0–2.0)* | 1.5 (1.0–2.75) |
Figure 1
Histopathological alterations in ovarian sections of the control and the experimental groups. A – control, B – smoke exposure, C – room air + 10 mg/kg melatonin, D – room air + 20 mg/kg melatonin, E – smoke exposure +10 mg/kg melatonin, F – smoke exposure + 20 mg/kg melatonin groups. G–L – higher magnification of the follicles in these groups, respectively. A–F – magnification 4×, G–L – magnification 20×, H&E)
CF – cystic follicle, DF – degenerated follicle, CL – corpus luteum; arrow, apoptotic granulosa cells in antrum.
Primordial follicles were found to be significantly different between the smoke exposure group and other groups. The number of primordial follicles was significantly reduced only in the smoke exposure group in comparison with the control group (p = 0.002). In the smoke exposure + 10 mg/kg melatonin group, the number of primordial follicles was significantly lower than the control group (p = 0.03) whereas there was no statistically significant difference between control group and smoke exposure + 20 mg/kg melatonin group.
Primary follicle counts were significantly decreased in the smoke exposure group compared to the control group (p = 0.006). The number of primary follicles was found to be significantly increased in the smoke exposure + 20 mg/kg melatonin group in comparison with the smoke exposure group (p = 0.006). Primary follicles were significantly fewer in the smoke exposure + 10 mg/kg melatonin group than in the control group (p = 0.047).
Secondary follicle counts were significantly reduced in the smoke exposure group compared to the control group (p = 0.004). The number of secondary follicles was found to be significantly increased in the smoke exposure + 20 mg/kg melatonin group in comparison with the smoke exposure group (p = 0.020).
Immunohistochemical analysis
Caspase-3 immunoreactivity
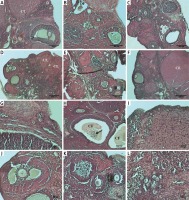
HSCORE was calculated by immunohistochemical analysis with anti-caspase 3 and determining the staining intensity of ovarian cells in all groups.
The staining intensity of the cells determined by the immunohistochemical applications with anti-caspase 3 is shown in Figure 2 and HSCORE values of the groups are presented in Figure 2.
Figure 2
A – Representative anti-caspase-3 immunoreactivity in ovarian sections of the control and experimental groups: a – control, b – smoke exposure, c – room air + 10 mg/kg melatonin, d – room air + 20 mg/kg melatonin, e –smoke exposure + 10 mg/kg melatonin, f – smoke exposure + 20 mg/kg melatonin groups, g–l – higher magnification of the follicles in these groups, respectively (a–f – magnification 10., g–l – magnification 20.). B – HSCORE of caspase-3 immunostaining in all groups
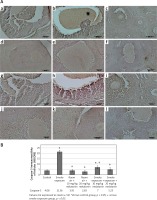
HSCORE was determined to be significantly increased only in the smoke exposure group (HSCORE = 21.3). HSCOREs of the smoke exposure + 10 mg/kg and smoke exposure + 20 mg/kg melatonin groups were found to be significantly reduced. Immunohistochemical analysis with anti-caspase 3 demonstrated an increased number of intense immunoreactive cells only in the smoke exposure group, whereas immunoreactive cells were found to be significantly reduced with decreased intensity in the smoke exposure + 20 mg/kg melatonin group. The immunoreactive cells were found to be lowered in the smoke exposure + 10 mg/kg melatonin group in comparison to the smoke exposure group, although not as effectively as in the smoke exposure + 20 mg/kg melatonin group.
TUNEL
The images acquired by the TUNEL method that was applied to the ovarian sections of the control and experimental groups are shown in Figure 3. The apoptotic index ratios (AIR) calculated by the TUNEL method are shown in Figure 3.
Figure 3
A – TUNEL-fluorescein analysis of ovarian follicles in control and experimental groups: a – control, b – smoke exposure, c – room air + 10 mg/kg melatonin, d – room air + 20 mg/kg melatonin, e – smoke exposure + 10 mg/kg melatonin, f – smoke exposure + 20 mg/kg melatonin groups. Apoptotic cells are indicated in green and shown with white arrows (a–f – magnification 10.). B – Apoptotic index of ovarian follicles in all groups
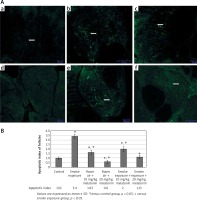
According to immunofluorescence images acquired by the TUNEL method, apoptotic cells were stained in large numbers and intensely in the smoke exposure group while apoptotic cells were found to be relatively small and decreased in intensity in the smoke exposure + 20 mg/kg melatonin group. Apoptotic cells were found to be relatively small and decreased in intensity in the smoke exposure + 10 mg/kg melatonin group as well, although not as effectively as in the smoke exposure + 20 mg/kg melatonin group.
Discussion
Approximately 4000 chemicals have been defined to be present in cigarette smoke (CS) and its negative impact on ovarian reserve has been demonstrated in various studies [8]. Gannon and his colleagues exposed rats to cigarette smoke twice a day for 5 days/8 weeks using a whole-body smoke exposure system [24]. Similarly, Tuttle et al. exposed rats to cigarette smoke twice a day for 7 days/8 weeks [18]. In both studies, it was concluded that ovarian volumes of the animals and the primary follicle numbers were significantly lower in the smoke exposure group. The mechanism of the effects of smoking is not precisely known although the negative effects of smoking on the ovarian reserve and follicle counts are inevitable according to literature data [1]. The literature data indicated that the primordial follicle has been particularly sensitive to toxic agents in primate models [25–27]. Our study indicated that the ovarian reserve is definitely impaired and the primordial follicle, primary follicle and secondary follicle numbers were significantly reduced by cigarette smoke.
In experimental studies evaluating the negative effects of smoking, two different models have been described in the literature: nose-only exposure (NOE) and whole-body smoking exposure [8, 9]. In the model where the rats are exposed to nose-only smoking, inhalation is achieved by special mechanisms and dermal exposure is less common. Two separate studies of Sobinoff et al. which were designed in this way revealed that the primordial follicle activation was performed after dimethylbenzanthracene (DMBA) application [6, 7]. From this point of view, as in our study, rats are placed in a device leading to whole body smoking exposures involving all nasal, oral and dermal routes.
Smoking is one of the main sources of exogenous pro-oxidants and smoke contains a large amount of free oxygen radicals [19]. As a result of increasing ROS levels in blood after smoking, the balance between pro-oxidants and anti-oxidants shifts in favor of pro-oxidants, resulting in oxidative stress [28]. Literature data have shown that both enzymatic and nonenzymatic antioxidant levels are reduced in cigarette smokers [29]. In those studies, antioxidant capacity of follicular fluid was found to be low. Reactive oxygen species in the maturation stage and the final maturation process at the diplotene stage increase their developmental potential by ensuring the reactivation of meiotic oocytes. Like the free oxygen radicals, high levels of NO produced by oocytes or follicular somatic cells play an important role in oocyte maturation and physiology. High free oxygen radicals in the environment due to reduced NO levels can cause injury at the cellular level [30, 31]. Calcium ions (Ca2+) accelerate the structural changes in the inner mitochondrial membrane and increase the free oxygen radical levels, leading to dysfunction of the mitochondrial respiratory chain.
Direct or indirect effects of melatonin on antioxidative enzymes brought into question its use as a powerful antioxidant. Its extremely low toxicity property, being able to be found in pure form, being relatively easy to obtain and not being expensive, have clearly expanded its use. The role of melatonin in reducing oxidative stress is stimulation of glutathione (GSH, a potent antioxidant) synthesis, reducing electron escape from the mitochondrial electron transport chain (reducing free radical formation), limitation of cytosine production of inflammatory processes (reduction of toxic reactant formation), and it has a synergistic effect with conventional antioxidants such as vitamin C, vitamin E or glutathione (GSH) [29]. In addition, melatonin inhibits H2O2-induced cell apoptosis by reducing the cytosolic free calcium concentration ([Ca2+]c). Melatonin, which is located directly in the follicular fluid (FF), can contribute to the development and reaching the fertilization stage of the preovulatory follicle.
In our study, melatonin given in room air significantly increased tissue levels of SOD, GPx and CAT. Tissue levels of CAT have been shown to be increased significantly in smoke exposure + 10 mg/kg and 20 mg/kg. Melatonin administered in room air was concluded to significantly induce the antioxidant pathways. 20 mg/kg melatonin treatment in the smoke exposure group was demonstrated to increase the levels of SOD, GPx and CAT in ovarian tissue, suggesting that those enzymes act more effectively in ovarian tissue. Serum levels of SOD, GPx and CAT were found to be higher in the group receiving 20 mg/kg melatonin in comparison with the 10 mg/kg melatonin group, although it was not statistically significant, suggesting that the enzymes work more effectively in serum with 20 mg/kg melatonin administration as well. In our study, serum and tissue levels of antioxidant parameters (SOD, GPx, CAT) were found to be decreased in the smoke exposure group whereas the same parameters were shown to be significantly increased in the smoke exposure + melatonin groups. Additionally, serum and tissue levels of MDA were significantly higher in the smoke exposure group in comparison with the control group whereas the same parameters of the smoke exposure + 20 mg/kg melatonin group were found to be similar to the control group, thus demonstrating that melatonin use at this dose significantly reduces the oxidant levels, achieving an antiapoptotic effect. In the smoke exposure + 20 mg/kg melatonin group, the number of follicles was found to be similar to the control group, thus indicating that melatonin exerts a protective effect for the ovarian follicles. Lastly, apoptotic indices detected by TUNEL and HSCORE results obtained by caspase-3 immunohistochemical analysis show similarities between smoke exposure + 20 mg/kg melatonin and control groups, suggesting positive effects of melatonin on apoptosis.
In our study, smoking was shown to increase apoptosis with respect to apoptotic indices and HSCOREs. Immunohistochemical analysis by anticaspase-3 and TUNEL immunofluorescence studies led us to conclude that there is a negative impact of smoking on ovarian reserve. Increased apoptosis detected in the ovarian stroma by TUNEL images of immunofluorescence staining confirmed this conclusion.
Duration might be one of the limitations of our study different from the literature. Tuttle et al. exposed rats to cigarette smoke for 8 weeks, which is different from our study protocol [18]. Our study is an animal study, which might be another disadvantage as physiology might not always reflect human physiology. One of the advantages of our study is that exposure intensity was more concentrated in a shorter time period, which might indicate dose-related effects. The way of exposure might be another advantage. Normally, the nasal exposure model investigates the local effects when compared to whole body exposure. Gannon and colleagues used the whole-body smoke exposure system, similar to our study [24]. In this way, it is possible to investigate the systemic effects of smoking beneath nasal effects.
The results of our study indicate a relation between smoke exposure and decline in ovarian reserve. Independent from way of exposure, either nasal or systemic, smoke exposure diminishes ovarian reserve. It is not clear whether this effect is reversible with antioxidant treatment. Different doses of melatonin might have a beneficial effect for the rejuvenation of ovarian reserve even though the exact mechanism is not known.
In conclusion, dose-related use of melatonin in smokers may be associated with a decreased apoptotic index and induced antioxidant activity in tissue in rat models. In our study, primordial follicle, primary follicle and secondary follicle numbers were significantly reduced because of cigarette smoking. That is why a decrease in follicle counts can reveal that ovarian reserve diminishes due to the negative effects of smoking, although melatonin which has antioxidant properties, should alter such adverse effects of smoking.