Introduction
Invasion and metastasis of tumour are mainly responsible for poor prognosis of cancer patients. Peritoneal metastasis accounts for about 50% of postoperative recurrence in gastric cancer. The exfoliation of exfoliative cells from gastric serosa into the peritoneum is the main cause of peritoneal metastasis. It has been reported that the positive rate of exfoliative cells in serosa infiltrated gastric cancer (T3) was only about 50%, but the majority of infiltrated gastric cancer were without exfoliative cells [1–4]. The differential expression levels of multiple genes and cytokines may account for the differences of gastric cancer cells in biological properties.
Previous studies have shown that the expression of vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) was correlated with growth, invasion and metastasis of gastric cancer, and increased ability of invasion and metastasis facilitated the growth and seeding of exfoliative cells in the peritoneum [5–10]. The differences in the expression of VEGF and EGF between positive and negative exfoliative cell gastric cancer tissues were investigated in order to study the exfoliative cells in the abdominal cavity that are related to peritoneal seeding in gastric cancer. The relationships between the ability of proliferation, migration, invasion and adhesion, and mRNA levels of EGF, VEGF and their respective receptors in four gastric cancer cells were examined. The effects of endostatin (Endostar) or cetuximab (Erbitux) blocking VEGF and EGF signalling pathways on the growth, migration, adhesion and invasion of a gastric cancer cell line with the highest level of EGF, VEGF and their respective receptors were also studied.
Material and methods
Patients and gastric cancer cell lines
Eighty patients with serosa-infiltrated gastric cancer were operated on at the Gastrointestinal Department. None of the patients had received radiotherapy, chemotherapy or immunotherapy. They were pathologically diagnosed as adenocarcinoma. Sixty-four cases had distal-end gastric cancer and the others had proximal-end gastric cancer. Patients consisted of 44 males and 36 females aged from 30 to 81 years (mean age: 58 years). Informed consent was obtained from all the patients. Ethic approval was obtained from the Medical Ethics Committee. According to the results of irrigation of the peritoneal cavity, the patients were categorized into two groups: exfoliative cytology positive and negative groups.
Gastric cancer cell lines BGC823 (poorly differentiated gastric adenocarcinoma), MGC803 (poorly differentiated gastric adenocarcinoma), HGC27 (undifferentiated gastric adenocarcinoma) and SGC7901 (moderately differentiated gastric adenocarcinoma) were provided by the Cancer Research Institute, Harbin Medical University. The cell lines were cultured in RPMI-1640 medium containing 10% foetal bovine serum (FBS), 100 U/ml Penicillium and 100 U/ml Streptomycin and 0.25% Parenzyme (GIBCO, USA), at 37°C, in 5% CO2, and 95% air.
Sample collection and exfoliative cytology analysis
Before intraperitoneal operation, the gastric bed, spleen fossa and pelvic cavity were washed with 1 l of physiologic saline at 37°C and were vacuumed out after gentle churning. The collected washing solution was centrifuged at 2000 rpm at 4°C for 10 min. The precipitate was resuspended with 1 ml of diethylpyrocarbonate water for 10 min before centrifugation at 2000 rpm for 3 min. After removing the supernatant which contained akaryocyte and peritoneal mesothelial cells, the precipitate was used for smear preparation for haematoxylin and eosin staining and CK18 immunohistochemical staining (Zymed, USA). The false positive and the false negative rates were examined by peritoneal lavage cytology and CK18 examination. The remains were defined as intra-abdominal exfoliative cells positive.
Survival rate and clinicopathological parameters
The survival rates were compared under clinicopathological parameters including exfoliative cytology, lymphatic node metastasis, infiltration of serosa and differentiation.
mRNA expression of EGF, EGFR, VEGF and VEGFR
Total RNA was extracted using Trizol (GIBCO, USA) from samples collected from patients or gastric cancer cell lines according to the protocol provided by the manufacturer. The extracted RNA was reverse-transcribed into cDNA using an RT-PCR kit (TaKaRa RNA PCR Kit (AMV) ver. 3.0, TaKaRa, Japan). Briefly, the reverse transcription (RT) reaction mixture contained 2 µl of MgCl2, 1 µl of 10×RT buffer, 3.75 µl of RNase free dH2O, 1 µl of dNTP (10 mM each), 0.25 µl of RNase inhibitor, 0.5 µl of avian myeloblastosis virus (AMV) reverse transcriptase, 0.5 µl of random 9-mers and a 1 µl sample RNA in a 10 µl final reaction volume. RT was performed in the following conditions: 30°C for 10 min, 42°C for 30 min, 99°C for 5 min, 5°C for 5 min and at –20°C for storage. The PCR mixture contained 20 ng each of primer, 2.5 µl of 10×PCR buffer, 0.4 µl of dNTPs (10 mM each), 0.1 µl of Taq polymerase and 40 µg of cDNA in a 25 µl final reaction volume. PCR was performed in a GeneAmp PCR system 9700 (Applied Biosystems, CA) with 94°C (1 cycle) for 2 min and 94°C for 30 s, 58°C for 30 s and 72°C for 1 min (30 cycles), with β-actin as the reference gene. The band density was quantified using Image J 1.38 software from the National Institute of Health.
MTT assay
Cell viability was assessed by the uptake of MTT (thiazolyl blue tetrazolium bromide; Sigma Chemical). Briefly, 6 × 103 gastric cancer cells in RPMI-1640 medium with 10% FBS were plated into 96-well plates. At 12, 24, 48, 72 or 96 h, the culture medium in each well was substituted with 200 µl of fresh medium containing MTT (final concentration, 250 µg/ml). Plates were then incubated for an additional 4 h period at 37°C. Subsequently, the medium was carefully removed with no disturbance to loosely adherent cells. Cells containing the trapped MTT crystals were then solubilized in 100 µl of dimethyl sulfoxide (DMSO) and vortexed for 10 min to dissolve the crystal thoroughly. Absorbance was determined in a microtitre plate reader (Molecular Devices, Menlo Park, CA) at 570 nm. MTT is a yellow-coloured dye. Living cells in the mitochondrial succinate dehydrogenase can metabolize MTT by the action of isopropyl alcohol particles. In the usual case, the amount of production is proportional to the number of viable cells, so the number of living cells can be deduced from the OD value of 570 nm.
Wound healing assay
Wound healing assay was used to detect the alteration of cell motility. Gastric cancer cells were seeded onto 35-mm plates at a density of 2 × 106 cells. When cells spread all over the plate, cells were cultured in serum-free medium for 24 h. Cells in half of the plate were erased using cell slicker. Photomicrograph was taken immediately (time 0 h), so that the migrated cells could be observed and microphotographed at 12, 24, 36 and 48 h. The experiment was performed in triplicate in each assay and was repeated four times.
Cell adhesion assay
Cell attachment to matrix (Matrigel) was carried out. Briefly, 8 g of Matrigel was coated to 96-well plates and incubated at 37°C for 1 h, 4 × 105 cancer cells were plated in Matrigel-coated plates in triplicate. After incubation at 37°C in 5% CO2 for 2 h, culture media were removed, followed by two washes with phosphate buffered saline. Cells remaining attached to the plate were analysed using MTT assay: % cells, attached = OD (attached cells)/OD (plated cells) × 100%.
Cell invasion assay
Invasion of gastric cancer cells was analysed using transwell culture chambers (SIGMA, Germany). Polycarbonate microporous membrane facies interna of transwell chambers was coated with 5 µg (10 µl) of Matrigel (SIGMA, Germany). Briefly, 5 × 104 gastric cancer cells were added to coated membranes in 100 µl of serum-free RPMI1640 medium containing 0.1% BSA in triplicate wells. 500 µl of RPMI1640 with 5% FBS medium were placed in a lower chamber and incubated for 24 h. After 24 h at the end of the experiment, the chambers were washed twice with 0.9% NaCl and submerged in 24-well plates in 10% FBS medium, then incubated at 37°C for 4 h in 5% CO2. The transwell was taken out and the filter membrane was fixed with methanol for 1 min. The cells that had not penetrated the membrane were erased with cotton swab. The cells were stained in haematoxylin for 3 min, then in eosin for 10 s. The invasive cells were counted under a microscope (400×). All invasion assays were done in triplicate in three independent experiments to ensure consistency. Representative fields were photographed.
Effect of Endostar or Erbitux
The expressions of EGF, EGFR, VEGF and VEGFR in MGC803 cells were evaluated using semi-quantitative PCR. The MGC803 cells were treated with 1.75 mg/ml Endostar (Endostatin, Simcere-Medgenn BioPharmaceutical Co., Ltd, Shandong, China) or 162 mg/ml Erbitux (Cetuximab, Merck-China, Beijing, China) for 12 h. The drug concentration is calculated according to this formula:

Erbitux (Cetuximab, Merck-China, Beijing, China) for 12 h was evaluated using semi-quantitative PCR. To test the effect of 1.75 μg/ml Endostar or 162 μg/ml Erbitux on the proliferation of MGC803 cells, MGC803 cells (6 × 103/well) were plated in a 96-well plate, followed by 4 h incubation at 37°C. Then, 1.75 μg/ml Endostar or 162 μg/ml Erbitux in 0.9% NaCl was added to MGC803 cells, with 0.9% NaCl as a negative control and oxaliplatin as a positive control. The proliferation was evaluated using MTT assay. To measure the effect of Endostar or Erbitux on the ability of migration, adhesion and invasion, MGC803 cells were plated into a culture flask, followed by 24 h incubation. Then, 1.75 μg/ml Endostar or 162 μg/ml Erbitux in 0.9% NaCl was added to culture medium for 12 with 0.9% NaCl as a control. The attached cells were used for migration, adhesion and invasion assays.
Results
Survival rate and clinicopathological parameters
Tables I and II show the clinical characteristics of the patients. The survival rate of exfoliative cytology positive cases is lower than negative cases (p < 0.01). Lower survival is related to lymphatic node metastasis, serosa-infiltrated and poorly differentiated gastric cancer (p < 0.01) (Figures 1 C–F).
Table I
Correlation of expressions of EGF in gastric cancers tissues with serosa invasion (T3) with clinicopathological parameters
Table II
Correlation of expression of VEGF in gastric cancer tissues with serosa invasion (T3) with clinicopathological parameters
Figure 1
mRNA expression of VEGF and EGF in cancer tissues and survival curves. A – mRNA levels of VEGF and EGF in cancer tissues from the patients with positive exfoliative cytology or negative exfoliative cytology. B – The quantification of band density (mean ± SD). mRNA levels of VEGF and EGF were normalized to the β-actin mRNA level. *P < 0.05. C – Survival curves of exfoliative cytology positive cases and negative cases. D – Survival curves of different lymphatic node metastasis cases. E – Survival curves with serosa-infiltrated cases and without serosa-infiltrated cases. F – Survival curves of poorly differentiated, well-moderately differentiated gastric cancer

mRNA levels of EGF and VEGF in gastric cancer tissues
The mRNA expression of EGF and VEGF was detected in gastric cancer tissues from 80 cases. Among of them, 57 cases are cytology positive and 23 cases are cytology negative. mRNA levels of EGF and VEGF in gastric cancer tissues from the cytology positive group were 1.5-fold and 2-fold higher, respectively, compared with the cytology negative group (Figures 1 A, B).
Proliferation, migration, adhesion and invasion ability of gastric cancer cells
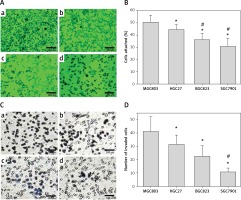
The ability of proliferation, migration, adhesion and invasion was assessed in four gastric cancer cell lines: BGC823 (poorly differentiated gastric adenocarcinoma), MGC803 (poorly differentiated gastric adenocarcinoma), HGC27 (undifferentiated gastric adenocarcinoma) and SGC7901 (moderately differentiated gastric adenocarcinoma). The growth rate of MGC803, HGC27, BGC823 and SGC7901 slowed down sequentially (Figure 2 A). The wound healing assay was carried out to test the migration ability of four gastric cancer cell lines. MGC803 showed the strongest migration ability and the migrated MGC803 cells to scraping wound was 2–3-fold higher than the migrated SGC7901 cells (Figure 2 B). The adhesion assay showed that more MGC803 cells were attached to the Matrigel than HGC27, BGC823 and SGC7901 (MGC803 vs. HGC27, BGC823 or SGC7901, 50.65 ±5.63 vs. 44.6 ±3.98, 36.22 ±5.10 or 30.83 ±6.64) (Figure 3 A). MGC803 showed the strongest ability of invasion to the matrix and invasion of MGC803 cells through Matrigel, which were 4-fold more numerous than the invaded SGC7901 cells (Figure 3 B). This suggests that MGC803 cells possess more invasive properties compared with HGC27, BGC823 and SGC7901.
Figure 2
Growth and migration ability of cancer cell lines. A – Growth curve of MGC803, HGC27, BGC823 and SGC7901. B – Representative micrograph of wound healing from MGC803 (a), HGC27 (b), BGC823 (c) and SGC7901 (d) at 36 h (e). Numbers of migrated cells were quantified at 12 h, 24 h, 36 h and 48 h (mean ± SD)
*P < 0.05 compared with MGC803 at the same time point, #p < 0.05 compared with HGC27 at the same time point.
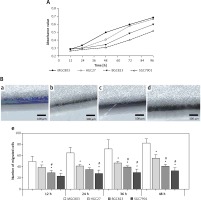
Figure 3
Adhesion and invasion ability of cancer cell lines. A – MGC803 (a), HGC27 (b), BGC823 (c) and SGC7901 (d) were tested for adhesion to a 96-well Matrigel-coated plate. B – Quantification of the adhesion ability (mean ± SD). *P < 0.05 compared with the MGC803. #P < 0.05 compared with HGC27. C – Matrigel invasion assay: the representative histogram of invaded MGC803 (a), HGC27 (b), BGC823 (c) and SGC7901 (d). D – Number of invaded cancer cells is quantified. *P < 0.05 compared with the MGC803, #p < 0.05 compared with HGC27 or BGC823
mRNA levels of EGF, EGFR, VEGF and VEGFR in gastric cancer cell lines
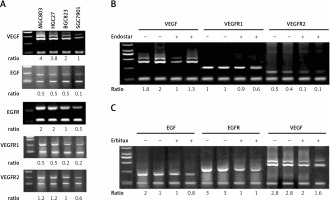
To examine the role of EGF and VEGF in advanced cancer, mRNA levels of EGF, EGFR, VEGF and VEGFR were examined in four gastric cancer cell lines. The results showed that VEGF, EGF, EGFR, VEGFR1 and VEGFR2 mRNA were expressed more highly in the MGC803 compared with the rest of the gastric cancer cell lines. The mRNA levels of VEGF, EGF, EGFR, VEGFR1 and VEGFR2 in MGC803 cells were 2–5-fold higher than the SGC7901 cells (Figure 4 A).
Figure 4
mRNA expression of VEGF and EGF in cancer cells with or without Endostar or Erbitux treatment. A – mRNA levels of VEGF, EGF, EGFR, VEGFR1 and VEGFR2 in colon cancer cells: MGC803, HGC27, BGC823 and SGC7901. B – VEGF, VEGFR1 and VEGFR2 in MGC803 cancer cells treated with Endostar. C – VEGF, EGFR and EGF in MGC803 cancer cells treated with Erbitux. Quantification of band density is shown. VEGF and EGF mRNA levels were normalized to the β-actin mRNA level
Inhibition of proliferation, migration, adhesion and invasion ability
MGC803 cells were treated with 1.75 μg/ml Endostar or 162 μg/ml Erbitux for 12 h to assess the effect of Endostar and Erbitux on the mRNA expression of VEGF, EGFR1, EGFR2, EGF, and EGFR. The results showed that Endostar decreased the VEGF, EGFR1 and EGFR2 mRNA levels in MGC803 cells by 39%, 25% and 78%, respectively. Erbitux decreased EGF, EGFR and VEGF mRNA levels by 40%, 67% and 64%, respectively. These data suggested that Endostar and Erbitux interrupted VEGF and EGF signals by decreasing the mRNA levels of EGF, VEGF and their receptors.
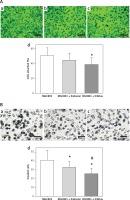
To further assess the role of EGF and VEGF in the proliferation, migration, adhesion and invasion ability, MGC803 cells were treated with 1.75 μg/ml Endostar or 162 μg/ml Erbitux (Figures 4 B, C). Both Endostar and Erbitux decreased the growth of MGC803 (Figure 5 A). After Endostar or Erbitux treatment (12, 24, 36 and 48 h), the migrated MGC803 cells to the scraping wound were decreased by 26%, 42%, 48% and 52%, respectively, compared with the vehicle-treated MGC803 cells (Figure 5 B). The ability of adhesion and invasion of MGC803 was decreased by Endostar or Erbitux. The attached MGC803 cells treated with Endostar or Erbitux were decreased by 20% or 22%, respectively, compared with the vehicle-treated MGC803 cells. Endostar and Erbitux decreased the invaded MGC803 cells through Matrigel by 22% and 39%, respectively (Figure 6). The inhibition of Endostar and Erbitux on the mRNA expression of VEGF, EGF and their receptors in MGC803 cells contributed to the decrease of the proliferation, migration, adhesion, and invasion ability of MGC803 cells.
Figure 5
Inhibitory effect of Endostar and Erbitux on the growth and migration ability of MGC803 cancer cells. A – Growth curve of MGC803 treated 0.9% NaCl as control or 1.75 mg/ml Endostar or Erbitux in 0.9% NaCl. B – Wound healing assay: representative micrograph of 36-h wound healing from MGC803 treated with 0.9% NaCl as control (a), 1.75 mg/ml Endostar (b) or Erbitux (c) in 0.9% NaCl for 12 h. Numbers of migrated cells were quantified at 12 h, 24 h, 36 h and 48 h (mean ± SD) (d)
*P < 0.05 compared with the MGC803 at the same time point.
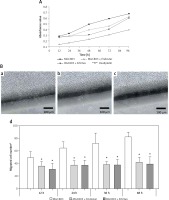
Figure 6
Inhibitory effect of Endostar and Erbitux on the adhesion and invasion ability of MGC803 cancer cells. A – MGC803 treated with 0.9% NaCl as control (a), 1.75 mg/ml Endostar (b) or Erbitux (c) in 0.9% NaCl for 12 h were tested for adhesion to a 96-well Matrigel-coated plate (d). Quantification of adhesion ability (mean ± SD). *P < 0.05 compared with MGC803. B – Matrigel invasion assay: representative histogram of invaded MGC803 (a), MGC803 treated with 1.75 mg/ml Endostar (b) or Erbitux (c) in 0.9% NaCl for 12 h and number of invaded cancer cells is quantified in (d). *P < 0.05 compared with the MGC803, #p < 0.05 compared with the BGC823 treated with Endostar
Discussion
EGF binds to receptors which are located on the cell membrane of various kinds of epithelial cells. EGF can stimulate the phosphorylation transduction pathway to regulate the proliferation and apoptosis of tumour cells. Many studies have revealed that the expression of EGF and its receptors was related to the ability of invasion and metastasis of gastric cancer cells, where overexpression signified short survival time and higher risk of recurrences for evaluation of prognosis [11, 12]. VEGF played a key role in the growth, invasion and metastasis of the tumour cells. VEGF binding receptors on the surface of vascular endothelial cells regulate angiogenesis of tumours and promote growth of tumours.
The results showed that lower survival was related to positive exfoliative cytology, lymphatic node metastasis, serosa-infiltrated and poorly differentiated gastric cancer. The biological properties of gastric cancer cells were related with prognosis. The mRNA expression of EGF and VEGF was significantly different between the exfoliative cytology positive cases and the negative cases. Two factors were confirmed to have a close correlation with abdominal cavity exfoliation of gastric cancer cells. As for serosa-infiltrated cases with cytology negative, overexpression of EGF and VEGF enhanced invasion and motility of the tumour cells to exfoliate into the abdominal cavity.
Contrarily, decreased secretion of EGF and VEGF inhibited proliferation of tumour cells, diminished nourishment and reduced metabolic capability of cancer cells. The opportunity of exfoliation of gastric cancer cells dropped accordingly. The mRNA levels of EGF, VEGF and their receptors in four gastric cancer cell lines showed that EGF and VEGF might regulate the biological properties of gastric cancer cells in an autocrine manner. Our study revealed that the properties compromised in order. This was in accordance with the expression of EGF, VEGF and their receptors in gastric cancer cells. Previous research has confirmed that the properties mentioned above were significantly correlated with peritoneal metastasis of gastric cancer [13–15]. Therefore, when EGF and VEGF were overexpressed in tumour cells, the function of transduction was stronger, which led to increased migration, invasion of gastric cancer cells and the likelihood of exfoliation. When exfoliated cells adhered to the peritoneum, EGF, VEGF and other factors increased the ability of proliferation, invasion and adhesion of cells. The tumour cells invaded the peritoneum easily, which led to micrangium genesis, provision of nutrition and implantation metastasis.
Vascular endostatin is hydrolysed from the carboxy terminal of extracellular matrix heparin sulfate glycoprotein (collagen XVIII). Its mechanism is still unclear. However, it is certain that it plays a key role in the process of tumourous angiogenesis. It can block activation of tyrosine phosphorylation, extracellular signal regulatory protein kinase, mitogen-activated protein kinase of KDR/Flk-1 (receptor of VEGF) and signal transduction mediated by VEGF [16]. Endostar can inhibit activities of matrix metalloprotease-9 (MMP-9) precursor and MMP-13 precursor, inhibit ability of peroxidase and the breakage of MMP-2 precursor [17], inhibit migration of endothelial cells and movement of endothelial cells, and promote apoptosis of endothelial cells [18, 19]. MGC803 was chosen as the experimental object as it showed the strongest expression of VEGF and other factors. When Endostar was added to MGC803, mRNA expression of VEGF and VEGFR was attenuated. It was likely that the blocked transduction of VEGF led to increased protein of free VEGF and free VEGFR, and negative feedback suppression of VEGF and VEGFR synthesis at the mRNA level.
Erbitux is an EGFR antagonist, and is also the only permitted new type of human/murine monoclonal enqomphesis antibody of EGFR to be used in clinic. Erbitux can inhibit proliferation of cancer cells, which leads to apoptosis of cancer cells, and decreases production of MMP and VEGF [20, 21]. In addition, the expression of cyclin D1 and HIF would be down-regulated, which made cells arrested at the G1/G0 stage with inhibition of tumour angiogenesis [22, 23]. In this study, the mRNA expression of EGF and EGFR in MGC803 cells treated with Erbitux for 12 h was obviously reduced. Erbitux blocked EGF transduction and inhibited synthesis of EGF and EGFR at the mRNA level, which was the same as the mechanism of Endostar. The mRNA level of VEGF in cells was reduced, which indicated that EGF can induce mRNA expression of VEGF. Erbitux inhibition of both EGF and VEGF showed that Erbitux was more effective towards MGC803 cells than Endostar in vitro.
Although oxaliplatin is more effective in the inhibition of MGC803 than Endostar and Erbitux, Endostar and Erbitux significantly inhibited the growth of MGC803 compared with vehicle-treated MGC803 cells, especially in 24 h or 72 h treatment. A previous study reported that inhibiting the expression of VEGF and its receptor by down-regulating MMP could inhibit tumour angiogenesis and tumour metastasis [24]. Nevertheless, another study found that the stimulating effect of VEGF165 on expression and invasion of MMP-9 in human leukemic cells could be blocked by antibodies of VEGFR-1 and VEGFR-2 [25]. It was likely that reduced function of EGF and VEGF could lead to the changes of expression and activities of factors related to peritoneal metastasis such as MMP and β-integrin.
In conclusion, this study confirmed that the effects of EGF and VEGF were closely correlated with the biological properties of gastric cancer cells. Targeted inhibition of EGF and VEGF not only can lead to growth suppression of primary tumour but also can inhibit the invasion and metastasis of gastric cancer cells. The present study offered a theoretical foundation for wide application of molecular-targeted therapy in advanced gastric cancer.


