Introduction
Subdural effusion is a common complication that occurs after decompressive craniectomy. Skull defects are a possible cause of subdural effusion, though the pathogenesis of the condition is unclear [1–3]; therefore, the treatment plan can be uncertain. Skull repair shows a good therapeutic effect on subdural effusion, and this technique has grown in popularity as a treatment for this condition. From January 2018 to March 2020, 19 cases of skull repair and treatment of subdural effusion were performed. During the operation, neuroendoscopy was performed to observe and treat the subdural effusion, and good curative effects were achieved. According to the endoscopy results, the formation mechanism of subdural effusion after decompressive craniectomy was discussed.
Aim
To observe the morphological structure of subdural effusion in skull defects with endoscopy, and endoscopic-assisted surgery performed for subdural effusion.
Material and methods
General information
Neurosurgery patients treated in Beijing Boai Hospital from January 2018 to March 2020 were analyzed retrospectively. These cases met the diagnostic criteria for postoperative subdural effusion [4–6]. Furthermore, 32 cases of subdural effusion were treated with skull repair. In 19 patients, neuroendoscopy was performed during the operation to observe and treat the subdural effusion, and these patients were assigned to the experimental group. Thirteen patients with subdural effusion were treated with a routine operation without intraoperative neuroendoscopy and were assigned to the control group.
Experimental group
Patients in this group included 14 male patients and 5 female patients, all aged between 31 and 71 years. Seven patients presented with craniocerebral injury, 11 patients had cerebral hemorrhage; 1 had bleeding induced by aneurysm rupture; 1 had tension of the bone window, which was slightly depressed, and the rest of the 19 patients were swollen; 14 had complications associated with hydrocephalus, 8 had effusion under the skin flap alone, 5 had complications associated with contralateral subdural effusion; 3 had effusion in the longitudinal fissure cistern; and 2 had effusion at the margin of the bone window.
Control group
Patients in this group included 11 male patients and 2 female patients, all aged between 19 and 64 years. Eight patients had craniocerebral injury; 3 had cerebral hemorrhage; 2 had bleeding induced by aneurysm rupture; 1 had tension of the bone window, which was slightly depressed, and the rest of the 13 patients were swollen; 5 had complications associated with hydrocephalus, 6 had effusion under the skin flap alone; 3 had complications associated with contralateral subdural effusion; 2 had effusion in the longitudinal fissure cistern; and 3 had effusion at the margin of the bone window.
Surgical methods
Experimental group
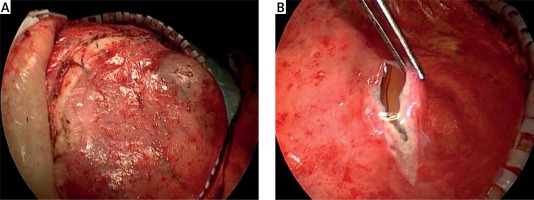
The scalp was cut along the original incision until the skull was reached. The skin flap was stripped from the dura and the dura was kept intact. If a rupture occurred, the dura was closely sutured, as shown in Photo 1 A. The dura was cut open, as shown in Photo 1 B. Under an endoscope, the subdural effusion capsule was inspected, and the structure of the inner and outer walls and boundary of the capsule were observed for possible arachnoid break. Inspection deep into the rupture was conducted to determine whether there was a residual cavity after the operation and whether the residual cavity was connected with the ventricle. Following this, the old hematocele or necrotic brain tissues were moved. A hemostasis gauze (Surgicel, Johnson & Johnson) was placed between the two layers of the subdural effusion cavity. If the arachnoid membrane was ruptured, the hemostasis gauze was placed at the site of the cerebrospinal fluid fistula under an endoscope. The dura was sutured and further occluded with swine-derived fibrin glue. The skull was repaired using titanium alloy or PEEK material. When subdural effusion in the contralateral side of the defect displaced from the middle line, it was punctured to drain the effusion. After the effusion had decreased, cranioplasty was performed; alternatively it was completed at the same time as the cranioplasty. No treatment was given for the effusion in the longitudinal fissure cistern and the effusion at the edge of the bone window. After the effusion disappeared, ventriculoperitoneal shunting was performed to treat the hydrocephalus patients.
Observation indexes
Dynamic reexamination of head computed tomography (CT) or magnetic resonance imaging (MRI) was performed within 1–2 months after cranioplasty. The observance of the midline as being located in the middle and the disappearance of effusion was defined as an effective result. If the effusion had not disappeared after 1 month, this was defined as an ineffective result (Table I). The approximate time when the effusion disappeared was recorded, and the clinical manifestations of the patients, including hydrocephalus, incision infection, intracranial infection, postoperative hemorrhage, epileptic attack, and effusion under the flap, were observed (Table II).
Table I
Comparison of surgical efficiency between the two groups
| Variable | Efficiency of subdural effusion | Postoperative effusion under flap | Infection rate |
|---|---|---|---|
| Experimental group | 18/19 | 2/19 | 1/19 |
| Control group | 12/13 | 6/13 | 2/13 |
| Statistic | p = 0.655a | p = 0.038a | p = 0.552a |
Results
Endoscopic observation of subdural effusion
Under an endoscope, all cases of subdural effusion in skull defects presented capsules. The main structures included the outer wall, boundary, inner wall, and fistula. The outer wall consisted of new tissue growing under the skin flap, after removing the bone flap, which was formed by artificial peeling under the condition of keeping the whole cavity. The endomembrane of the outer wall was smooth; in some cases, two layers of incompletely closed gaps could be observed (Photo 2 A). The inner and outer wall of the skull were fused to form the boundary of the cavity (Photos 2 B, C). The inner wall consisted of thickened arachnoid, dura, and artificial dura and presented with neovascularization networks, which showed a tendency to form new membrane structures through mutual adhesion and fusion (Photo 2 C). In some cases, the inner wall of the cystic cavity formed an intact, uniform, and smooth structure; dead cavities formed in 5 patients; and no cerebrospinal leakage was found (Photo 2 B). Clear fistulas were observed in 6 patients (Photo 2 E). In 3 patients, the fistula was connected directly with the ventricle or indirectly with the ventricle through the residual cavity. A post-operative residual cavity was found below the fistula in 3 patients. Old hematocele was found in the residual cavity in 1 patient (Photo 2 F). No clear fistula but a continuous exudation of cerebrospinal fluid was found in 8 patients. The closure of the structure of the fistula as a result of the thickened arachnoid was seen in 1 patient (Photo 2 G).
Photo 2
Endoscopic observation of the cavity structure of subdural effusion: A – the incompletely closed boundary of the cavity, 1 – the incompletely closed holes on the boundary, 2 – the thickened arachnoid covering the brain surface, 3 – the upper and lower layers of adhesion of the cavity, 4 – the hyperplastic vascular network on the arachnoid; B – completely closed cavity boundary, 1 – inner wall, 2 – outer wall, 3 – boundary line; C – fusion of arachnoid and artificial dura mater, 1 – adhesion, 2 – proliferative vascular network, 3 – artificial dura mater; D – contralateral subdural effusion of defect and thickened arachnoid without boundary or leakage; E – arachnoid fistula structure, 1 – dura mater, 2 – thickened arachnoid, 3 – brain tissue without arachnoid cover, 4 – leakage, connected with residual cavity after the operation, indirectly connected with ventricle, 5 – normal arachnoid, 6 – outer wall of cavity; F – residual cavity hematoma; G – fistula completely closed by arachnoid, 1 – dura mater, 2 – hyperplastic adhesion zone and vascular network, 3 – fistula completely covering by thickened arachnoid; H – filling hemostasis gauze in the fistula or between inner and outer walls
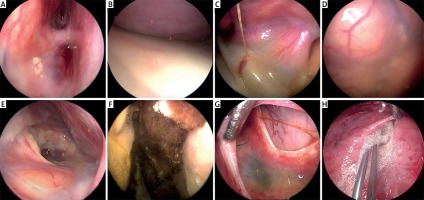
Contralateral subdural effusion of skull defects was observed by endoscopy in 1 patient. The effusion was located under the dura mater without cavity formation, a thickened arachnoid was found on the surface of the brain, and no cerebrospinal fluid fistula was found (Photo 2 D).
Under an endoscope, a hemostasis gauze was carefully placed in the fistula or between the two inner and outer walls to prevent the liquid from leaking (Photo 2 H).
Comparison of operation results between the two groups
Experimental group
Sixteen patients’ skulls were repaired with titanium alloy material, and 3 patients’ skulls were repaired with PEEK material. CT reexamination revealed that effusion under the flap disappeared within 6 h following the operation in 15 patients, within 3 days following the operation in 2 patients, within 10 days following the operation in 1 patient, and within 35 days following the operation, indicating an effective result, in 1 patient. Contralateral subdural effusion was found in 5 patients, 2 of whom underwent puncture and drainage; effusion disappeared at 25 days following the operation in 1 patient; 3 days following the operation in 1 patient; and 6 h following the operation in 3 patients. Effusion was found in the interhemispheric fissure in 3 patients and disappeared within 6 h following the operation in 2 of these patients and 3 days following the operation in the remaining patient. Effusion was found at the margin of the bone window in 2 patients but disappeared within 6 h following the operation. Hydrocephalus was found in 14 patients, 12 of whom underwent ventriculoperitoneal shunt (VPS) at the second stage. In 10 of these patients, the ventricles became smaller because of the release of effusion and subsequently became gradually larger following the repair, leading to a more severe condition than had been the case before the operation. At 7–21 days following the repair, a second operation was performed. Two patients’ symptoms were aggravated within 1 week due to intolerance of hydrocephalus, and these patients underwent drainage of the lumbar cerebellomedullary cistern to temporarily relieve the symptoms before undergoing VPS as soon as possible. One patient exhibited mild hydrocephalus that was not aggravated in follow-up appointments, and in 1 patient, the ventricles recovered to their normal size and remained normal at a 1-year follow-up. Two patients had effusion under the flap after repair, and 1 patient had intracranial infection after VPS.
Control group
Nine patients had skull repair with titanium alloy material, and 4 patients had skull repair with PEEK material. In 10 patients, an incision was made at the dura mater to release the effusion. This was sutured only, and peeling was conducted along the original dura. Incomplete skull repair was performed using artificial dura mater in 1 patient; 1 patient underwent puncture of the residual cavity to release fluid, and the partial inner wall of the effusion cavity was peeled to connect the effusion cavity with the subarachnoid cavity in 1 patient. CT reexamination revealed that effusion under the flap disappeared 6 h following the operation in 11 patients, at 13 days following the operation in1 patient, and over 1 month after the operation in1 patient, which indicated an ineffective result. Contralateral subdural effusion was found in 3 patients and disappeared 3 days following the operation in 1 patient and 6 h following the operation in 2 patients. Effusion was found in the interhemispheric fissure in 2 patients and disappeared 6 h following the operation in 2 patients. Effusion was found at the margin of the bone window in 3 patients and disappeared 6 h following the operation in all 3 patients. Hydrocephalus was found in 5 patients; of them, 4 underwent VPS at the second stage. The ventricles recovered to their normal size and remained normal at the 1-year follow-up in 1 patient. Six patients exhibited effusion under the flap after repair.
Discussion
The pathogenesis of subdural effusion after decompressive craniectomy remains unclear [1–3, 7, 8], and no single theory can fully explain all the clinical manifestations. There are two main theories for the mechanism of subdural effusion. In the first theory, the arachnoid is ruptured as a result of external force, and the cerebrospinal fluid flows into the subarachnoid space and the subdural space through the rupture opening. The arachnoid at the ruptured opening may form a unidirectional valve, or if the rupture opening is blocked by blood clots, cerebrospinal fluid continuously flows out during the breath holding, coughing and other movements of injured patients, but cannot return to the subarachnoid space. This eventually leads to accumulation of fluid under the dura. If a patient has a large volume of effusion, this can produce adverse consequences, such as local brain tissue compression and progressive intracranial pressure increase. In the second theory, the destruction of the blood-brain barrier occurs for a variety of reasons, leading to an increase in capillary permeability. Plasma exudates lead to subdural effusion, and the high osmotic pressure of the effusion gradually leads to the infiltration of water into the surrounding tissues and subarachnoid space, thus increasing the volume of the effusion. The imbalance of intracranial pressure, the vibration of brain tissue, the change in the circulation power of the cerebrospinal fluid and the change of osmotic pressure are relevant factors in subdural effusion after decompressive craniectomy [9–14].
Endoscopes are useful in skull repair because they are not blocked by the margin of the bone, which enables the effusion cavity and the depth of the fistula to be observed from different angles. The contralateral effusion of the defect is observed through a single bone hole. Due to the limitation of the bone hole, the whole image of the effusion cannot be observed by a hard endoscope. The formation mechanism of effusion remains in theoretical speculation and has no objective basis. Endoscopic observation of the shape and structure of the subdural effusion constitutes an objective basis from which to consider the formation mechanism of subdural effusion.
The effusion cavity can be divided into three types based on its structural characteristics: fistula type, membrane type, and closed type. Fistula type: as shown in Photos 2 E and F, the essence of the fistula is the defective arachnoid. Significant fistulas were detected in the post-operative cystic cavity (destruction of brain tissue caused by brain injury or cerebral hemorrhage, formed by removal of necrotic tissue or hematocele) or in the ventricles. No fistula with valve was found in this study. Membrane type: there was no obvious fistula, though cerebrospinal fluid continuously appeared in the effusion cavity after suctioning the effusion. The membrane structure could be seen under an endoscope. The arachnoid sealed the fistula (Photo 2 G), was connected with dura mater (Photo 2 C), and the thickened arachnoid structure was accompanied with vascular hyperplasia (Photo 2 A). There are several possible reasons that could explain the lack of obvious fistula, though cerebrospinal fluid appeared continuously in the effusion cavity. There may have been a fistula that was not found; the fistula was too small and could not be distinguished by endoscopy, and the possibility of complete penetration was not excluded; however, the rapid appearance of effusion suggested that the effusion entered the capsule through penetration. Closed type: this refers to a dead cavity (Photo 2 B) in which the intima is thickened to show the homogeneous structure of the wall; there is no fistula or membrane structure, and no new effusion after suctioning the effusion.
It is speculated that the mechanism of formation of subdural effusion is as follows: in the early stage, there is an arachnoid fistula. The presence of a fistula, fluctuations in the cerebrospinal fluid, and the uneven distribution of intracranial pressure after decompressive craniectomy cause the effusion cavity to gradually enlarge. Fluid accumulates in the lacunae under the flap and outside the dura mater, the presence of liquid promotes membranization of the surface of the skin flap in contact with the liquid, and the arachnoid thickens, adheres to the dura or artificial dura and grows. Because of the bulge of brain tissue, it presses on the edge of the bone window and the effusion is limited under the flap, and the two layers of the effusion cavity fuse to form a bounded cavity (Photos 2 A, B). After pressure balancing, the fistula may be covered or incompletely covered by hyperplastic arachnoid membrane, there may be a fistula that is not found, or there may be penetration. Finally, the fistula is completely closed to form a dead cavity (i.e., a closed type). Therefore, it is speculated that the main cause of effusion is skull defects and arachnoid membrane damage; the fistula type-membrane type-closed type process is a continuous process of gradual evolution; skull defects lead to the formation of the effusion boundary. Exudation may be present, but is not a primary factor.
Subdural effusion under flaps in skull defects differs from effusion that occurs in the side opposite to the defect [15, 16] and in the longitudinal fissure cistern. It also differs from non-surgical subdural effusion after trauma. In this study, there was no cavity formation, contralateral effusion was observed in only1 patient by endoscopy and only the thickening of the arachnoid and the effusion under the flap could be seen. A few of these had no formed cavity, and the effusion did not disappear after repair. This is known as “refractory effusion”, and its mechanism and treatment require further study.
The structure of the effusion cavity was observed by endoscopy. The inner surface of the outer wall of the effusion was smooth, while the outer surface was rough. A new dura formed artificially in the process of the sharp separation of the skin flap. The inner wall of the effusion was formed by adhesion and fusion of thickened new arachnoid membrane and the original dura mater or artificial dura mater. In skull defects with subdural effusion, anatomically and strictly speaking, effusion was not located in the subdural, but rather in the epidural space. Following skull repair, the outer wall of the effusion was under the bone flap, forming the so-called new dura. The effusion also became a subdural effusion.
Peeling off the outer wall of the effusion to achieve a true anatomical reduction of the skull is inadvisable. Comparing the two groups revealed that this increased the incidence of effusion under the flap following the operation. Maintaining the integrity and sealing of the outer wall of the effusion (that is, the new dura mater) was key in reducing the leakage of cerebrospinal fluid. In addition, the upper and lower layers of tissues easily grew through the repair material and connected with the mesh of the repair material. Peeling off this layer of membrane made the repair material contact with the dura mater and the thickened arachnoid. There may be a fistula that is not found, and the growth time of the two layers of tissues with the repair material was prolonged; both factors can increase the risk of effusion under the flap. Therefore, the occurrence of skin flap effusion was effectively reduced by keeping the outer wall of the subdural effusion cavity intact.
Endoscopic treatment of residual cavity hematocele was simple and quick, and no complication associated with bleeding or infection occurred. However, whether this treatment can promote the recovery of brain function is not known. Placing hemostasis gauze at the fistula and in the effusion cavity is designed to promote the adhesion of the two layers. There was no difference in the effective rate between the two groups. Previous literature has shown skull repair to be an effective method for treating subdural effusion [2, 4, 6]. The results of this study revealed that skull repair was the main factor for eliminating effusion. However, the time for effusion disappearance was faster in the experimental group. This suggests that placing hemostatic gauze in the cavity and fistula is beneficial, and its mechanism is similar to that of puncture treatment of subdural effusion. But if the puncture treatment effect is poor, then it turns into chronic subdural hematoma. If the effect of puncture is good, hematoma may cause adhesions in the effusion cavity [17–19]. This represents a new treatment option for refractory subdural effusion, though its efficacy requires further verification in future studies.
Conclusions
Hydrocephalus with subdural effusion is common [20–24]. Most cases are characterized by the disappearance of effusion and aggravation of hydrocephalus after repair. As the effusion cavity is directly or indirectly connected with the ventricle, releasing the effusion during the operation can shrink the brain ventricles and reduce the tension in the brain. The bulged brain tissue subsequently reenters the bone window to enable the skull repair to be successfully completed. Simultaneous repair and shunt placement are not conducive to the disappearance of effusion. Staged surgical treatment can reduce the infection factors of shunt operations, such as skin flap effusion (cerebrospinal fluid fistula), and infection at the surgical incision [25]. Although only 2 patients with hydrocephalus were relieved after repair, this suggests that repair may reduce hydrocephalus and even enable shunt operations to be avoided, which is one of the significance factors of staging [26].