Introduction
Pseudoaneurysm (PSA) is defined as a pulsating encapsulated haematoma that develops in connection with the lumen of a ruptured vessel after an injury to all layers of the arterial wall. The lack of a 3-layer structure distinguishes it from a true aneurysm, and it makes sudden rupture more probable. The incidence of common carotid artery PSAs (CCA-PSA) of all extracranial aneurysms is 0.1-0.3% [1,2].
The aim of treating CCA-PSA is to avoid rupture, embolic complications, and neurological disability associated with the procedure. The treatment choices include surgery and endovascular therapy [3]. Traditionally, surgery was the treatment of choice, but due to improved equipment availability, the new endovascular techniques provided a less invasive solution with increased efficacy.
Case presentation
A 45-year-old-male labourer presented with a steadily growing pulsatile mass on the left side of his neck, which had been present for 2 months. On detailed retrospective interrogation, he gave a history of a high-speed shrapnel injury while working at a construction site. Following this, he experienced a minor injury to the neck that healed with the help of first aid treatment. Physical examination showed a painless, pulsatile mass on the left neck region. The neurological test was normal and his vital signs stable.
The multi-detector computed tomography (MDCT) scan revealed the presence of 2 different PSAs, one larger in size and fusiform in shape, and the other smaller sized, oval-shaped from the posterolateral and anteromedial walls of the common carotid artery, respectively (Figure 1). A selective carotid angiogram showed 2 PSAs as described earlier from the distal segment of left CCA below the bifurcation with an appropriate landing zone (Figure 2).
Figure 1
Multidetector computed tomography angiography of the left carotid artery. Maximum intensity projection images in sagittal section (A) and coronal section (B) showing 2 separate pseudoaneurysms, a largesized, fusiform-shaped (solid white arrow) along the posterolateral wall and another small-sized, oval-shaped along the anteromedial wall (dashed white arrow) arising at the same level from the distal segment of the left common carotid artery below the bifurcation
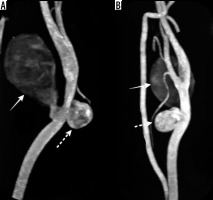
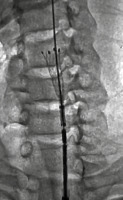
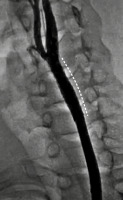
Figure 2
Selective angiography of the left common carotid artery. Pseudoaneurysms showing in 2 different phases of filling, (A) partially and (B) near-complete filling phases due to slow, phasic filling secondary to their narrow necks (black asterisks; a). It demonstrated 2 separate pseudoaneurysms: a largesized, fusiform (solid white arrow) and another small-sized, oval-shaped (dashed white arrow) arising at the same level from the left common carotid artery below the bifurcation
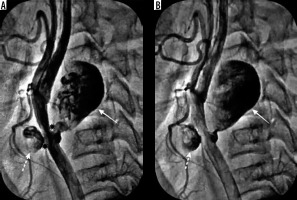
The endovascular intervention of the PSA first involved the placement of a 0.035-inch guidewire (Terumo Corporation, Japan) via the femoral approach into the left internal carotid artery across the left CCA using an 8F long-sheath. We decided to treat the patient with a stent graft [covered stent]; hence, a 25 × 7 mm Viabahn (Gore and associates, USA) self-expanding covered stent was then expanded in the CCA across both of the PSAs leading to their complete closure (Figures 3 and 4). The patient was discharged with dual antiplatelet therapy (aspirin 150 mg and clopidogrel 75 mg) on the 2nd day post procedure. Patients had 3, 6, and 12 months of clinical follow-up, and each time carotid Doppler was performed, showing a patent stent without PSA recurrence or endoleak. Thereafter, he was recommended for an annual check-up.
Discussion
Traumatic CCA-PSAs, although rare, can be life-threatening and difficult to treat. Blunt, penetrating, or iatrogenic injuries may result in traumatic extracranial carotid PSAs. Penetrating carotid artery injuries secondary to high-velocity fragment injuries occur in up to 10% of all neck trauma cases and carry a high mortality rate of up to 50% if remains unmanaged [2,4].
MDCT has been used as a diagnostic imaging modality in most institutions because it is non-invasive, widely available, and has high sensitivity and specificity. Biffl et al. suggested a method of grading these traumatic neck injuries. The PSAs were considered as grade III lesions as they arise from full-thickness wall damage, resulting in a localized partially perfused haematoma [5].
Management of extracranial carotid lesions depends on the extent of the lesion and the overall general condition of the patient, from surgical treatment like open repair to endovascular treatment like deployment of a covered stent. Because of the reduced invasiveness and improved procedural effectiveness, endovascular repair has received more acceptance [6]. The approaches for endovascular management rely on the age of the patient, the location of PSA, and other associated diseases of the cerebral circulation and the status of its collateral circulation. The location of the PSA, whether extra- or intra-cranial, affects the option of which hardware to use. Extracranial CCA is a simple anatomical structure, without any major branches [3].
Many previously described methods like coiling or stent-assisted coil embolization may not be successful if the source of bleeding is not addressed. The use of the covered stent remains the treatment of choice because it maintains the patency of the parent artery and results in early complete obliteration of the PSA [7-9]. Both the balloon and self-expanding covered stents available. It is now well established that balloon-expandable stents are not suitable for extracranial carotid arteries because they are subjected to increased external compression, which leads to serious damage and its occlusion [10].
Our option was to use a self-expanding, covered stent, which that is more durable and less likely to be compromised by external stress. For this reason, the Viabahn stent (Gore & Associates), which is a polytetrafluoroethylene (PTFE)-covered nitinol self-expanding stent, is well suited because the PTFE is nonporous and prevents type IV endoleak [2,11]. Hoppe et al. reported on their experience with carotid artery stent-grafts in 25 patients. Their rate of technical success was 100%. Procedural dissection and short-term complications were observed, respectively, in 7.4% and 11% of their patients without any long-term medical complications [12].
The post-procedure antithrombotic strategy is critical for long-term effectiveness, because the probability of stent occlusion in this setting has been documented to be much higher compared to atherosclerotic disease. For this reason, despite the high occurrence, no particular protocol was established, and the strategy of dual antiplatelet therapy with clopidogrel, and aspirin was recommended based on the experience [2,13]. Regular follow-up of the patient should be carried out using clinical examination and carotid Doppler at an interval of 3, 6, and 12 months initially and later annually. There is no clear established protocol, and clinicians should use their experience based on their institutional practices.
Conclusions
Carotid artery injury associated with high-velocity fragment trauma to the head and neck is a serious condition, but early diagnosis and successful treatment results in low morbidity and mortality. The effect of undisclosed lesions can be serious, and every effort should be made to screen all high-risk patients if there is any history of neck injury in the presence of neck swelling. Endovascular interventions offer a safe and reliable method of treatment for CCA-PSAs with the advent of improved hardware and skills; furthermore, management must be customized based on clinical and imaging profiles for each patient such as aneurysm location, characteristics, etc. Unfortunately, there are no clear guidelines in the current literature to suggest the appropriate plan of treatment due to its low incidence, limitations in the documentation of outcomes, and the inconsistency of indication. To specify the treatment options for CCA-PSA, a multicentre international registry is required. Also, postprocedural dual antiplatelet treatment and follow-up using ultrasound are crucial to increasing the likelihood of successful long-term outcomes.