Introduction
Gastric adenocarcinoma (GCA) is a common malignancy with a global prevalence. Lauren et al. classified GCAs into 3 broad categories on histopathology: intestinal (welldifferentiated), diffuse (poorly-differentiated), and indeterminate/mixed types [1]. Tumours in these categories exhibit differences in aetiology, genetics, and epidemiology [2,3]. Histological characterization is also one of the important prognostic factor in GCA [4]. The diffuse type is associated with an aggressive clinical course. Adachi et al. showed that the overall 5-year survival rate for patients with well-differentiated GCA was higher than that for patients with poorly-differentiated type (76% vs. 67%; p = 0.058), especially in patients with tumours ≥ 10 cm (42% vs. 14%; p = 0.017) [4]. Currently, histological characterization of GCA is possible only by endoscopic-guided biopsy. However, endoscopy is an invasive procedure and has its limitations. The obtained biopsy may not be representative of the entire tumour, which is known to show geographical histological variations [5]. Furthermore, the extramural extent of the tumour, and distant nodal and visceral metastases cannot be assessed by endoscopy.
Previous studies have attempted to differentiate histological types of GCA using computed tomography (CT) [6-11]. Tsurumaru et al. studied peak enhancement in GCAs using multiphasic contrast-enhanced CT gastrography (CE-CTG) [10]. They concluded that the 3 broad histological categories of GCA can be predicted by measuring the peak enhancement on a multiphasic CT. The purpose of our study was to examine the utility of multiphasic contrast-enhanced CT in differentiating the 3 broad histological categories of GCAs.
Material and methods
This was a prospective study approved by our institutional review board, and informed written consent was taken from all patients. Inclusion criteria included consecutive patients who underwent endoscopic-guided biopsy and showed histological evidence of GCA. Upper gastrointestinal endoscopy was performed in all 70 patients using an Olympus GIF-Q150 Actera. All 70 patients underwent subsequent multiphasic CT as part of clinical staging.
Histopathological classification
The tumours were graded into the following categories for our study: a) differentiated type, b) undifferentiated type, and c) mixed types. The following criteria were applied for the above classification. Differentiated type included well and moderately differentiated GCAs, and papillary adenocarcinomas. Undifferentiated type included poorly differentiated adenocarcinomas, signet-ring cell carcinomas, and mucinous variant. Mixed type were those with features of both differentiated and undifferentiated GCA.
Multiphasic computed tomography protocol
All 70 patients underwent multiphasic CT on a 128-slice multidetector scanner: SOMATOM Definition AS+, Siemens, Erlangen, Germany. Patient preparation included overnight fasting. To achieve adequate gastric distension, each patient was given 600-1000 ml of water as negative contrast immediately before the exam in the CT suite. We did not administer any anti-peristaltic agent to our patients. Pre-contrast axial images of the upper abdomen were acquired using the following parameters: slice thickness and intervals = 5 mm, rotation time = 0.5 s, pitch = 1.5, kVp = 100, mAs = 170, and matrix = 512 × 512. For triphasic post-contrast CT, 70-80 ml of nonionic contrast medium (Iopromide 370 mg/ml; Bayer AG, Germany) was administered intravenously using a power injector at the flow rate of 3.5-4 ml/s. Axial images of the upper abdomen were acquired during the arterial phase, the whole abdomen for portal-venous phase, and again the upper abdomen during the hepatic-venous phase using a kVp = 120, mAs = 210. The other acquisition parameters were similar to the pre-contrast scan. At our institution, the abdominal triphasic CT images are obtained at 21 s for arterial dominant phase, 32 s for portal-venous phase, and 50 s for hepatic-venous phase after the start of intravenous injection. The same timings were also applied for this study. We acquired axial images of the chest during the arterial phase to evaluate for lung metastases, but these images were not included for analysis. All axial acquisitions were reconstructed to 1 mm axial slice thickness. Coronal and sagittal reformations were reconstructed from the 1 mm axial reconstructions for all 3 phases.
Image interpretation
Two experienced radiologists in abdominal radiology reviewed the CT dataset. Both the radiologists were blinded to the histopathological findings but used endoscopic findings for reference. The following observations were recorded by the readers, and any disagreement was resolved by consensus.
Size of gastric lesion: The largest 2 orthogonal dimensions were recorded for each lesion by the readers and were averaged.
Tumour location: For the purpose of analysis, the stomach was anatomically divided into 3 portions: upper (U), middle (M), and lower (L) segments, by the lines connecting the trisected points on the lesser and greater curvatures [12]. Gastric tumours were classified by the segments involved as U, M, or L. If more than 1 segment was involved, the tumours were classified by the segment containing the bulk of the tumour. If all 3 segments were involved, the tumour was classified as diffuse (D). Oesophageal and duodenal extension of the tumours were also recorded as ES and DU, respectively.
Tumour peak-enhancement characteristics: The readers manually traced regions of interest (ROIs) at the maximum attenuation value (HU) for the pre-contrast and all 3 post-contrast phase images. The maximum values (HU) were averaged for the readers. The peak-enhancement phase for the tumours were classified as ‘arterial’, ‘portal-venous’, and ‘hepatic-venous’ based on the phase with the maximum attenuation value. The chi-square test was performed to determine the association of enhancement pattern between differentiated and undifferentiated tumours. Independent samples T-test was applied to determine the predictability of contrast study for differentiating the type of GCA.
Tumour, Node, and Metastasis staging: 8th American Joint Committee on Cancer (AJCC) staging was followed for staging purposes [13].
Results
The study included 70 patients who were positive for GCA after endoscopic-guided biopsy. The 70 patients included 47 males. Mean age was 52.5 years (SD ± 13.3). The tumour location, histopathology, and clinical TNM staging for the 70 patients are summarized in Table 1. Notably, there was no mixed type on histopathological analysis in our cohort. The tumour maximum dimensions varied between 2.7 cm and 12.0 cm. The majority of the tumours were predominantly located in the lower gastric segment in both the differentiated and undifferentiated types (n = 25/28 for differentiated GCAs and n = 27/42 for undifferentiated GCAs). Diffuse GCAs involving all 3 segments were seen only in the undifferentiated type (n = 3/42). All 70 GCAs in our series were staged ≥ IIB.
Table 1
Histopathological type, location, extent, and tumour staging for N = 70 with gastric adenocarcinoma
Peak-enhancement characteristic
The peak-enhancement type was ‘arterial’ in 20 out of 28 within the differentiated-type GCAs and ‘portal-venous’ in 37 out of 42 within the undifferentiated-type GCAs (c2 statistic with Yates’s correction = 23.3981, p < 0.00001) (Table 2). The mean of the maximum attenuation values (HU) for 70 GCA on multiphasic CT is summarized in Table 3. Mean maximum attenuation value (HU) was higher in the arterial phase for differentiated GCAs and in the portal-venous phase for undifferentiated GCAs. The maximum attenuation value was statistically significant for the arterial phase between differentiated and undifferentiated GCAs (p ≤ 0.05). There was no statistical significance for portal-venous (p = 0.08) and hepatic-venous (p ≤ 0.19) phases (Supplemental Table).
Table 2
Peak-enhancement pattern on multiphasic computed tomography for N = 70 gastric adenocarcinomas
| Histological type | Peak-enhancement type | |
|---|---|---|
| Arterial | Portal-venous | |
| Differentiated type, n = 28 | 20 | 8 |
| Undifferentiated type, n = 42 | 5 | 37 |
Table 3
Maximum attenuation value (HU) on multiphasic CT for 70 GCAs
There was no significant statistical difference in lymph nodal and hepatic metastasis between the differentiated and undifferentiated carcinomas in our study (c2 statistic with Yates’s correction = 1.7414, p = 0.186 for lymph node metastasis; c2 statistic with Yates’s correction = 0.0076, p = 0.930 for hepatic metastasis).
Discussion
Adachi et al. studied the microvascular architecture of GCA in relation to histologic subtypes. They observed that on histopathology, the differentiated carcinomas were frequently normovascular (65%) or hypervascular (24%), whereas undifferentiated carcinomas were hypovascular (60%). They believed that stromal hypervascularity in well-differentiated GCA may partly account for its propensity to metastasize to liver and lung [14]. However, in our study, we did not observe a statistically significant difference in hepatic metastasis between differentiated and undifferentiated GCAs (Table 1) (Yates’s correlation = 0.0076, p = 0.930).
The enhancement characteristics of GCAs have been a subject of interest in previous studies with implications on tumour detection and staging, and with reported differences among the histological categories[6-11,15-18]. Undifferentiated GCAs carry a poor prognosis. Hence, the ability to predict histology based on CT characteristics may add clinical value. In our study, there was a statistically significant difference in the enhancement pattern between differentiated and undifferentiated GCAs. An arterial phase dominant enhancement was present in 71.4% of the differentiated GCAs based on the maximum attenuation value measured within the tumour (Figures 2, 4, 5 and 8). A portal-phase dominant enhancement was observed in 88% of undifferentiated GCAs (Figures 1, 3, 6 and 7).Our observations again probably reflect the microvascular architecture of these tumours, as previously reported by Adachi et al. [14].
Figure 1
Typical undifferentiated: Undifferentiated-type gastric cancer in a 65-year-old woman. A-C) Contrast-enhanced computed tomography abdomen showed a 4.1 × 2.7 cm mass in the antropyloric region. Regions of interest (ROI) are drawn in the area having the highest attenuation value. The computed tomography enhancement peaked in the portal phase, and the attenuation values were 58 HU in the arterial phase and 85 HU in the portal phase. D) Photomicrograph (original magnification ×400) shows poorly differentiated adenocarcinoma
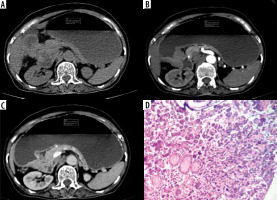
Figure 2
Typical differentiated: Differentiated-type gastric cancer in a 60-year-old man. A-C) Contrast-enhanced computed tomography abdomen showed a large 8.5 × 3.7 cm enhancing mass in the antrum (circle ROI). The computed tomography enhancement peaked in the arterial phase and the attenuation values were 72 HU in the arterial phase and 56 HU in the portal phase. D) Photomicrograph (original magnification ×100) shows differentiated adenocarcinoma
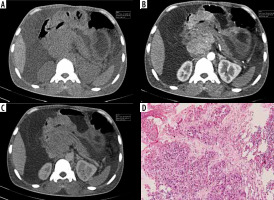
Figure 3
Atypical undifferentiated: Atypical enhancement pattern of undifferentiated-type gastric cancer in a 65-year-old woman. A-C) Contrast-enhanced computed tomography abdomen showed focal thickening measuring 5 × 4.6 cm in the posterior wall of the stomach (circle ROI). The computed tomography enhancement peaked in the arterial phase, and the attenuation values were 122 HU in the arterial phase, 88 HU in the portal phase. D) Photomicrograph (original magnification ×200) shows poorly differentiated adenocarcinoma with signet ring cells
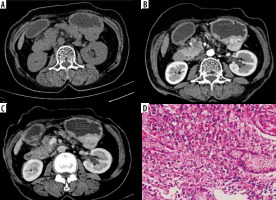
Figure 4
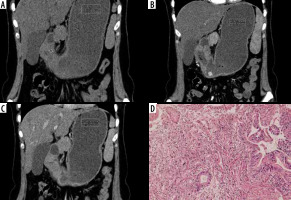
Atypical differentiated: Atypical enhancement pattern of differentiated-type gastric cancer in a 67-year-old woman. A-C) Contrast-enhanced computed tomography abdomen coronal MPR image showed a 6 × 3.8 cm mass in the antrum (circle ROI). The computed tomography enhancement peaked in the portal phase and the attenuation values were 47 HU in the arterial phase and 63 HU in the portal phase. D) Photomicrograph (original magnification ×100) shows differentiated adenocarcinoma
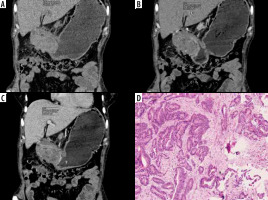
Figure 5
Typical differentiated: Differentiated-type gastric cancer in a 89-year-old man. A-C) Contrast-enhanced computed tomography abdomen showed focal thickening in the posterior wall of stomach (circle ROI). The computed tomography enhancement peaked in the arterial phase, and the attenuation values were 77 HU in the arterial phase and 57 HU in the portal phase. D) Photomicrograph (original magnification ×100) shows differentiated adenocarcinoma
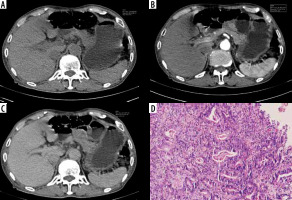
Figure 6
Typical undifferentiated: Undifferentiated type in a 43-year-old man. A-C) Contrast-enhanced computed tomography abdomen showed circumferential thickening in the antropyloric region (circle ROI) measuring 8.4 × 3.3 cm. The computed tomography enhancement peaked in the portal phase, and the attenuation values were 51 HU in the arterial phase and 68 HU in the portal phase. D) Photomicrograph (original magnification ×200) shows poorly differentiated adenocarcinoma-mucinous type
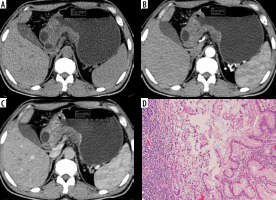
Figure 7
Atypical undifferentiated: Atypical enhancement pattern of undifferentiated-type gastric cancer in a 35-year-old woman. A-C) Contrast-enhanced computed tomography abdomen showed circumferential thickening in the antrum (circle ROI) measuring 6.7 × 2.4 cm in size. The computed tomography enhancement peaked in the arterial phase, and the attenuation values were 66 HU in the arterial phase and 56 HU in the portal phase. D) Photomicrograph (original magnification ×100) shows poorly differentiated adenocarcinoma
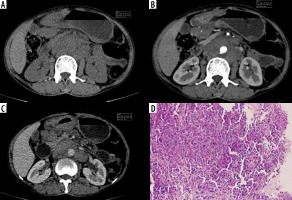
Figure 8
Atypical differentiated: Atypical enhancement pattern of differentiated-type gastric cancer in a 40-year-old woman. A-C) Contrast-enhanced computed tomography abdomen coronal MPR image showed a 8.1 × 3.1 cm mass in the antropyloric region (circle ROI). The computed tomography enhancement peaked in the portal phase and the attenuation values were 55 HU in the arterial phase and 76 HU in the portal phase. D) Photomicrograph (original magnification ×100) shows differentiated adenocarcinoma.
In a previous similar study, Tsurumaru et al. retrospectively examined 47 patients with advanced GCAs (T2-T4) using multiphasic CT with arterial, portal venous, and delayed-phase images at 40 s, 70 s, and 240 s, respectively. They reported statistically significant higher CT attenuation values in undifferentiated GCAs in the delayed phase compared to mixed and differentiated types (p = 0.004). Using a cut-off of 109.8 HU, the maximum accuracy for undifferentiated GCAs in the delayed phase in their study was 72.3% [10]. Contrary to our observations, they did not find significant difference in the arterial phase between the histological types. However, a direct comparison to their study may be limited due to differences in the CT protocol applied.
In a different study, Tsurumaru et al. retrospectively studied the enhancement pattern specifically in 21 diffuse-type advanced GCAs (histologically poorly differentiated adenocarcinomas) using dual-phasic CT [9]. They observed a double-layered pattern in all 21 cases in the arterial phase with a high attenuating inner layer. Such a pattern was not seen in our study. Also, there was no comparison with differentiated GCAs in their study and the mean attenuation value was employed for analysis rather than the maximum attenuation value.
Lee et al. studied the portal-venous phase enhancement characteristics in 81 gastric cancers (35 with signet ring cell carcinoma [SRC] and 45 non-signet ring cell carcinoma [NSRC]) [6]. They observed high-degree contrast enhancement (higher than liver) more frequently in SRC than in NSRC (37.1% vs. 15.6%, respectively) in the portalvenous phase. They attributed this to the presence of immature fibrosis in SRCs, which are considered a subset of diffuse GCAs. In our study, we did not observe a significant difference in enhancement pattern in the venous phase in the 8 cases of diffuse GCAs with signet ring histology compared to the rest.
Our study has limitations. First, this was a single-institution study with a relatively small sample size. Secondly, we only included the broad histological categories of GCA for our analysis and did not analyse the subtypes within these broad categories. Also, our cohort did not have mixed type for analysis. Thirdly, we only measured the maximum attenuation value within the tumour for analysis and did not consider the mean attenuation of the entire tumour. Given the known heterogeneity in tumours, this may not be a correct representation of the microvascular architecture. Finally, the definition of arterial and venous phases in our CT protocol is discordant with other previously reported studies, which makes direct comparison difficult.
Conclusions
Assessing peak enhancement in a multiphasic CT exam can help suggest the histological subcategory of gastric carcinomas, which has prognostic significance. Arterial phase peak-enhancement is frequently seen in differentiated carcinomas, whereas venous phase peak-enhancement in undifferentiated carcinomas. This is probably reflective of their microvascular architecture as reported in previous studies.


