The open abdomen technique (OA) is a surgical strategy used in life-threatening conditions such as those related to intra-abdominal bleeding, prevention or treatment of intra-abdominal hypertension and treatment of intra-abdominal sepsis. It is a temporary measure to stabilize patients with the goal of correcting metabolic and physiologic disorders, besides facilitating access to the abdominal cavities [1].
McGosh in 1897 was the first to describe the OA and the concept of damage control was described by Stone in 1983. Damage control (DC) as known today was conceived and first described in 1993 by Rotondo et al. as an alternative to definitive laparotomy in patients with exsanguinating hemorrhage related to large vessel lesions and multiple intra-abdominal viscera lesions [2]. Afterwards, it was demonstrated that initiating damage control surgery early, before the patient’s clinical conditions deteriorated to the extreme [massive loss of blood, severe trauma (Injury Severity Score; ISS > 25), hypothermia (< 34°C), acidosis (pH < 7.25), and coagulopathy (activated partial thromboplastin time; APTT > 19 s)], reduces mortality [1, 3]. Although OA carries a lot of benefit, like any surgical intervention by keeping the abdo-minal cavity open it exposes the patient to the risk of hollow viscera perforation and increases the risk of developing complex abdominal hernias. Temporary abdominal closure techniques (TAC) with skin suture or closure with Backhaus clamps reduce these complications, but increase the risk of abdominal compartment syndrome (ACS); these are not techniques recommended any more [4].
After recognizing the morbidity and mortality attributed to ACS, several methods were developed to avoid this complication [4, 5]. The ideal technique for TAC was defined as one that contains the abdominal viscera, limits contamination, prevents loss of abdominal fluid, prevents adhesions, allows easy access to the abdominal cavity, prevents damage and retraction of the abdominal wall and avoids enteric fistulas and the development of ACS [6, 7]. The application of the Bogota bag, developed by Oswaldo Borráez in 1984, has become the most popular and effective method of temporary abdominal closure [8]. It is still used in many hospitals in developing countries because of its low cost and easy handling.
A little more than a decade ago, Barker et al. introduced the concept of negative pressure application as a new modality of temporary abdominal closure [9]. Historically, the OA was treated with simpler approaches such as the Bogota bag [8], Wittmann patch [10] and Barker’s vacuum pack [9], which yielded a variety of complications such as marked adhesion formation, development of enteric fistula, non-quantifiable loss of fluids, evisceration, hemorrhage, contamination of the abdominal cavity surgical wound (especially when in proximity to stomas), spread of bacteria into the ICU and ward environment, and a high rate of subsequent ventral hernias [11]. Different methods of TAC have been developed to protect the temporary OA and decrease complications [12, 13]. The primary goal of TAC is to create a tension-free closure of the abdomen without increasing intra-abdominal pressure. The optimal method of TAC should contain and protect the contents of the peritoneal cavity from external contamination and injury, preserve fascia; minimize desiccation and damage to viscera, remove and quantify third space fluid; prevent loss of domain, lower bacterial count and the inflammatory response, keep the patient’s abdominal wall skin dry and intact; preserve the integrity of the abdominal wall, be simple to perform and maintain, provide ease of reentry and have minimal adverse physio-logic effects [4, 7, 13].
During the past two decades, there has been a paradigm shift toward management of patients with severe abdominal sepsis by OA as a viable alternative to the previously used scheduled repeat laparotomy or continuous peritoneal lavage [14], as to deal with or prevent recurrent infection. In addition, it is well established that the visceral or retroperitoneal edema secondary to shock and reperfusion may increase intra-abdominal pressure to dangerous levels, leading to intra-abdominal hypertension and organ dysfunction [15]. Patients with this constellation of symptoms must have their abdomens left temporarily open to allow for visceral and renal perfusion as well as adequate pulmonary function.
Negative pressure wound therapy (NPWT) allowed the TAC method to drain the peritoneal fluid, to minimize visceral edema, to eradicate ACS, to apply greater fascial tension in the abdominal wall and to promote definitive abdominal closure [16–18]. A major obstacle to abdominal closure is the retraction of the rectus abdominis muscles, which should be avoided at all costs as closing the abdomen is still a challenging task even for the most experienced surgeon. NPWT resulted in greater rates of fascia closure, obviating the need for subsequent hernia repair in many patients [19]. The utility of this technique is not limited to the early postoperative period, but can be successful for up to 3–4 weeks after the initial operation [4, 7, 20, 21]. Recent large scale studies have reinforced the benefits of NPWT as compared to other TAC methods, and its early application has been shown to be beneficial [20]. While the patient is with an open abdomen, the fascial edges must be tensioned by means of interrupted suture with heavy-gauge nonabsorbable suture or a mesh. This strategy avoids fascial retraction and facilitates the progressive approach of the aponeurotic borders at each reoperation until the definitive abdominal closure [22, 23].
Several techniques for the surgical management of the complex OA have already been described including for Björck grade IV with NPWT [24, 25]. Here we describe our current opinion on two new alternatives to manage the septic complex abdomen with entero-atmospheric fistula (EAF).
NEW MANAGEMENT ALTERNATIVES: MIGRATING ENTERO-ATMOSPHERIC FISTULAS
Treatment of EAF for septic complex abdomen remains a challenge even for the most experienced surgeon. NPWT in these cases is already established as state-of-the-art, but closing the abdominal fascia and treating the enteric fistula at the same time is not commonly seen [20, 26]. Ultimately acute care surgeons either choose for “donut technique” to treat EAF or NPWT dynamic fascia closure for open abdomens Björck grade 1 (A and B) and grade 2 (A and B) [4, 20, 25]. Management of complex septic abdomen using both NPWT for dynamic closure of the fascia and enteric fistula control is also possible [20].
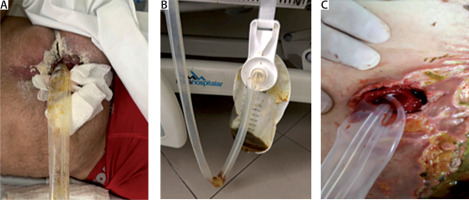
Here we describe the case of a young 32-year-old woman who underwent a complex gastric bypass procedure that required two interventions (the first on PO5 and second on PO9) due to severe bowel adhesions. On the last intervention an EAF was noted as seen in Figure 1A (Björck grade IIIb). On surgical exploration the bariatric surgery team isolated the fistula with a Foley catheter (Figure 1B). The acute care surgery (ACS) team was consulted to assist in the management of EAF and possible abdominal closure. A decision was made to use the “donut technique” to isolate the EAF over the cropped ABThera (Acelity 2018) (Figures 1C–E). The Foley catheter was kept in place with a port-o-vac drain circling both the inner part of the donut and the Foley exiting through the abdominal wall Figure 1E. This technique allowed the NPWT to work on the abdominal fascia with no leakage from the EAF (day 1). After placing the first ABThera blue foam over the star shaped bowel cover, a polypropylene mesh was sutured to the fascia as demonstrated in Figure 1F. A running suture right on the middle line of the mesh was performed, promoting tension to the fascia Figure 1G. Another ABThera blue foam was placed over the polypropylene mesh and negative pressure applied at 125 mm Hg (16,7 kPa). The patient was sent to the ICU intubated and was kept sedated and paralyzed with neuromuscular blockage in order to avoid abdominal hypertension. The leukogram started falling back to regular levels. Four days later the acute care surgery team took the patient back to the OR for the first ABThera NPWT change as seen in Figure 2A. Polypropylene mesh was opened at the middle line and another donut was manufactured (Figure 2B). NPWT was applied in the same way as before keeping both the Foley catheter and port-o-vac. Very little enteric leak was capture by the port-o-vac drain during these four days at the ICU. Abdominal fascia was continuously approximated as can be observed in Figure 2C.
FIGURE 1
A) Bogota bag (Björck grade IIIb), B) open abdomen with the fistula diverted with a Foley catheter, C) crafted donut, D–E) donut positioning, F–G) mesh mediated temporary abdominal closure (TAC), H) ABTheraTM TAC
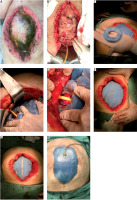
After another four days the ACS team reached the OR for the new ABThera, totaling 8 days of effective dynamic treatment of the abdominal fascia. EAF was still controlled by a Foley catheter into the fistula orifice and the port-o-vac drain outside the fistula Figure 3. In the OR, no contamination was observed (Figures 2E–F).
FIGURE 2
A) Open abdomen (OA) technique aspect after first mediated temporary abdominal closure (TAC) change. Note the open mesh held by surgeons. B) New crafted donut with foam stitched to the outer layer of the donut, which is in contact with the bowel. The outer layer foam lies over the star-shaped ABTheraTM mesh and promotes higher adherence to the peritoneum and subsequently better tissue granulation. C–D) Second mesh mediated TAC. E–F) Third mesh mediated TAC with better approach of the fascia and skin. G–H) Abdominal closure
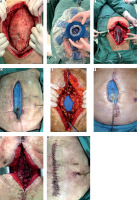
FIGURE 3
A) Enteric effluents through Foley catheter. B) Minimum enteric effluents through sentinel port-o-vac drain. C) Foley catheter and port-o-vac drain through the abdominal wall
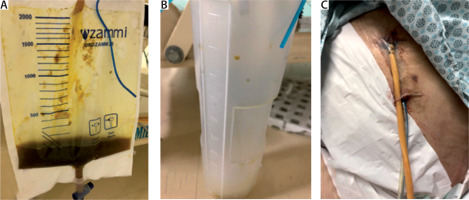
Four days more were left to a new intervention. At this stage the abdomen was pretty much clean and with granulation tissue. The Foley catheter was removed and non-absorbable sutures were placed at the fistula hole. The port-o-vac drain was kept guarding the former EAF and freshly sutured site. Abdominal fascia and skin were completely closed at the end of 12 days with total resolution of the EAF. The Port-o-vac drain was removed within the next four days. From the first surgical approach early parenteral nutrition was started at 2086 kcal/day and octreotide was prescribed at 100 µg subcutaneously every 8 hours for 10 days. Oral nutrition started after the third day of the last surgical intervention and on the tenth day after the last surgical procedure the patient was discharged home.
NEW MANAGEMENT ALTERNATIVES: ENTERO-ATMOSPHERIC FISTULA DIVERSION
Another new alternative that can be used in some cases is the off-label use of the Flexi-Seal (Conva-Tec) to diverge enteric effluents away from the skin on the frozen abdomen with EAF or collapsed colostomies.
The Flexi-Seal EAF control device was used in 5 consecutive patients with successful intestinal effluent diversion. This allowed the abdominal skin to recover faster in OA cases with EAF, besides allowing the ischemic/collapsed stoma to heal or save time for new intervention in critical patients allowing the dehiscent surgical wound communicating with the stoma to be treated in isolation with NPWT (Figure 3). It is important to stress that, to our knowledge, there are no clinical studies in the literature that corroborate the use of this device via a stoma, but its use in these cases is reasonable, caused no harm to the patients and the good results were remarkable.
CONCLUSIONS
One of the most devastating complications in the management of an OA is formation of an EAF. EAF appear especially in the course of prolonged OA management, which predisposes to the development of intra-abdominal adhesions and finally frozen abdomen. All manipulation of the fragile intra-abdominal contents, including NPWT temporary abdominal closure changes, is considered as a potential risk factor for iatrogenic or new bowel injury and thus increases risk of EAF formation [27].
The use of the donut inside the abdomen “excluding” the fistula site from the rest of the abdomen under NPWT was a new strategy adopted which allowed closure of the abdomen in 12 days by controlling EAF and migrating it from the midline to under the skin. Focusing on migrating enteric effluents outside the OA and transforming an EAF into an EF must be set as a priority.
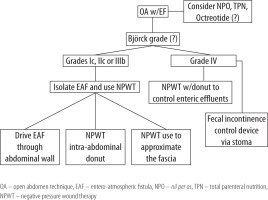
NPWT with continuous fascial traction has proven its role as an efficient means to achieve primary fascial closure after OA [20, 22, 26–28]. In 2014 a group of authors from Europe concluded NPWT to be the best option currently available to treat grade 3 OA with an EAF [29]. In the case presented here, the formation of a migrating EAF positioned laterally rather than the median line of the abdomen facilitated the intra-cavitary donut usage. We propose a treatment algorithm for the formation of a migrating fistula using the intra-cavitary donut or EAF diversion device via the stoma (Figure 5). The updated Björck classification is presented in Table 1 [30].
TABLE 1
Björck grading classification for the open abdomen technique