Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
HYPERTENSION / CLINICAL RESEARCH
Epidemiology of hypertensive heart disease in Poland: findings from the Global Burden of Disease Study 2016
1
Department of Hypertension and Internal Diseases, Pomeranian Medical University, Szczecin, Poland
2
School of Population and Public Health, University of British Columbia, Canada
3
Department of Cardiology, Pomeranian Medical University, Szczecin, Poland
4
Centre for Medical Simulations, Pomeranian Medical University, Szczecin, Poland
Submission date: 2018-05-19
Final revision date: 2018-09-13
Acceptance date: 2018-09-16
Online publication date: 2019-05-17
Publication date: 2021-07-16
Arch Med Sci 2021;17(4):874-880
KEYWORDS
hypertensive heart diseaseGlobal Burden of Disease Studyprevalencemortalitydisability-adjusted life years lost
TOPICS
ABSTRACT
Introduction:
Hypertension may cause target organ damage leading to hypertensive heart disease (HHD). The burden caused by HHD in Poland has not been studied systematically. The purpose of this study was to describe the burden of HHD in Poland in terms of prevalence, mortality, disabilityadjusted life years lost (DALY) and key risk factors.
Material and methods:
Data were obtained from the Global Burden of Diseases, Injuries and Risk Factors (GBD) Study database. The GBD uses a wide range of data sources and complex statistical methods to estimate disease burden for all countries by age, sex, and year. HHD was defined by ICD-9 codes 402-402.91 and ICD-10 codes I11-I11.9. From the GBD 2016 estimates, we extracted data for Poland between 1990 and 2016.
Results:
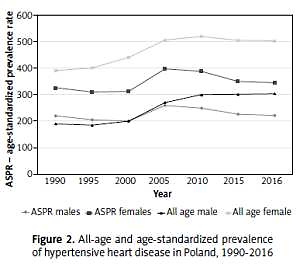
Hypertensive heart disease is the fourth most important cause of cardio- and cerebrovascular death, after ischemic heart disease, stroke and cardiomyopathy. In 2016, there were about 180 000 people diagnosed with HHD in Poland and close to 5000 HHD-related deaths. HHD prevalence increased from 0.29% in 1990 to 0.47% in 2016 and was higher in women, while mortality increased from 11.2 to 12.7 per 100 000, largely due to population aging. Age-standardized death and DALY rates declined between 1990 and 2016 and were lower than in Central Europe but higher than in Western Europe.
Conclusions:
Our data suggest a need for national initiatives to improve the diagnosis and treatment of hypertension, slow the progression of HHD, and reduce the related risks and premature deaths.
Hypertension may cause target organ damage leading to hypertensive heart disease (HHD). The burden caused by HHD in Poland has not been studied systematically. The purpose of this study was to describe the burden of HHD in Poland in terms of prevalence, mortality, disabilityadjusted life years lost (DALY) and key risk factors.
Material and methods:
Data were obtained from the Global Burden of Diseases, Injuries and Risk Factors (GBD) Study database. The GBD uses a wide range of data sources and complex statistical methods to estimate disease burden for all countries by age, sex, and year. HHD was defined by ICD-9 codes 402-402.91 and ICD-10 codes I11-I11.9. From the GBD 2016 estimates, we extracted data for Poland between 1990 and 2016.
Results:
Hypertensive heart disease is the fourth most important cause of cardio- and cerebrovascular death, after ischemic heart disease, stroke and cardiomyopathy. In 2016, there were about 180 000 people diagnosed with HHD in Poland and close to 5000 HHD-related deaths. HHD prevalence increased from 0.29% in 1990 to 0.47% in 2016 and was higher in women, while mortality increased from 11.2 to 12.7 per 100 000, largely due to population aging. Age-standardized death and DALY rates declined between 1990 and 2016 and were lower than in Central Europe but higher than in Western Europe.
Conclusions:
Our data suggest a need for national initiatives to improve the diagnosis and treatment of hypertension, slow the progression of HHD, and reduce the related risks and premature deaths.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.