Introduction
Obstructive jaundice (OJ) caused by benign or malignant diseases is common in clinical practice, and is often associated with several acute and chronic inflammatory reactions that in turn lead to morphologic and metabolic disorders [1–3]. Despite modern diagnostic and therapeutic approaches, OJ is still a clinical entity with up to 56% morbidity and 10–25% mortality rates due to septic complications [4]. Current clinical and experimental studies have demonstrated a correlation between OJ and the development of complications and sepsis, but the mechanism has not been fully elucidated [5, 6]. Two points play a key role in the pathophysiology of septic complications. One is physical disruption of the intestinal barrier function and the other is an impaired immune system [7, 8]. During OJ, the impairment of the intestinal mucosal barrier and the translocation of endotoxins and intestinal bacteria can cause intestinal infections and secondary multiple organ injury, which are pivotal for the deterioration of the disease [9, 10]. Therefore, it is of great significance to protect the small intestinal mucosa. Toll-like receptors (TLRs), a family of transmembrane receptors of the innate immune system, can non-specifically bind to a pathogen-associated molecule, initiate signal transduction, and eventually lead to activation of nuclear factor NF-κB as well as the expression of mediators. Therefore TLRs become the link between innate and adaptive immunity and play an important role in the intestinal mucosal barrier [11]. In recent years, with the discovery and functional identification of TLR family members, the role of TLRs has became a hot topic again in various etiologies and pathogenesis of intestinal diseases, such as inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) [12–14]. However, it remains unclear how TLRs function in the pathogenesis of OJ.
The present study was designed to investigate the expression changes of intestinal TLR2 and TLR5 in the process of experimental OJ, and to analyze their association with OJ, so as to explore their mechanism in the pathogenesis of OJ.
Material and methods
Animal models
C57BL/6J mice (n = 100; adult male; 20–24 g) were used in this study. All animals were purchased from the Laboratory Animal Center of Academy of Military Medical Sciences (License: SCXK (Jing) 2016–0006) and housed in the Experimental Animal Service Center of the Chinese PLA General Hospital (PLAGH). The animal use protocol had been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the PLAGH. Mice were fed with standard rat food before and after surgery. The animals were randomly placed in two groups: (I) sham operation (SH, n = 50); (II) bile duct ligation (BDL, n = 50). The mice in the BDL group had the common bile duct ligated above the pancreas, and then cut and sutured to establish the model of BDL on day 0. SH was performed with loose ligation of the common bile duct. All mice had an almost immediate and uneventful recovery from surgery and were returned to clean cages. Thereafter, their clinical status was observed daily. The mice were respectively sacrificed before the operation and on the 1st, 3rd, 5th and 7th days after operation to collect blood and terminal ileum specimens. All procedures were performed under anesthesia with 1.5% isoflurane and 98.5% oxygen using a delivery and scavenging system designed in our laboratory [15].
Liver function measurement
Blood samples were withdrawn from the inferior vena cava and centrifuged at a speed of 4,000 rpm for 10 min to isolate the serum, which was stored at –80°C until use. Serum aminotransferase (ALT), total bilirubin (TB) and alkaline phosphatase (ALP) were measured in the Biochemical Laboratory of the Chinese PLA General Hospital.
Histopathological examination
The samples of terminal ileum were fixed in 10% neutral formalin and embedded in paraffin. They were sectioned into 5 mm thin slices for staining with hematoxylin-eosin (HE). Microscopic examination was performed by a pathologist, who was blinded to the study. Chiu’s method was used for grading the mucosal injury [16]. The mucosal changes are graded as follows: grade 0: normal mucosal villi, grade 1: development of subepithelial Gruenhagen’s space, usually at the apex of the villus, often with capillary congestion, grade 2: extension of the subepithelial space with moderate lifting of epithelial layer from the lamina propria, grade 3: massive epithelial lifting down the sides of villi – a few tips may be denuded, grade 4: denuded villi with lamina propria and dilated capillaries exposed; increased cellularity of lamina propria may be noted, and grade 5: digestion and disintegration of lamina propria – hemorrhage and ulceration.
Intestinal flora detection
Content of the terminal ileum was collected and diluted 10 times after weighing on an electric scale. 10 μl of dilution was inoculated in corresponding bacterial culture medium and cultured for 48 h at 37°C. Gram staining was performed and colony numbers were counted, and then colony count per gram of feces (CFU/g) was calculated accordingly.
Semi-quantitative immunohistochemistry
Immunohistochemical staining was performed on sections of five randomly selected samples of the terminal ileum per animal. Briefly, 5 mm paraffin sections were deparaffinized in xylene and rehydrated in a gradient of ethanol solutions. Antigen retrieval was carried out by microwave heating the sections for 20 min in citric acid buffer, and then cooling for 15 min at room temperature (RT). Endogenous peroxidase activity was quenched with 3% hydrogen peroxide at RT for 10 min. Nonspecific binding was blocked by incubating the sections for 30 min in normal goat serum. The sections were incubated overnight at 4°C with anti-TLR2 antibody (rabbit polyclonal antibody against TLR2; ab213676, Abcam, UK) at a dilution of 1 : 400, anti-TLR5 antibody (rabbit polyclonal antibody against TLR5; ab62460, Abcam, UK) at a dilution of 1 : 100, the anti-NF-κBp65 antibody (rabbit monoclonal antibody against NF-κBp65; ab32536, Abcam, UK) at a dilution of 1 : 800. Following several rinses in phosphate buffered saline, the sections were incubated with anti-rabbit immunoglobulin G antibody tagged with horseradish peroxidase (BOSTER Biotechnology, Wuhan, China) for 30 min at 37°C. Finally, the sections were colored with diaminobenzidine at RT for 1–5 min, counterstained with hematoxylin for 5 min, dehydrated through an ethanol gradient, cleared in xylene, and then mounted with Permount. Images were obtained using a light microscope (CMS800; Olympus, Tokyo Japan). Immunoreactivity of TLR2, TLR5 and NF-κBp65 in mouse ileum was morphometrically identified by the Image Pro Plus 6.0 image analysis software system (Media Cybernetics, MD, USA) to determine mean optical density (MOD).
Real-time-PCR analysis
Quantitative analysis of TLR2 and TLR5 mRNA was performed by real-time-PCR. Total RNA was extracted by TRIzol reagent from Sigma. Purity and concentration of RNAs were determined by a NaNO2000 UV spectrophotometer and OD 260/OD 280 was within 1.8–2.2, which indicated the high purity of RNAs. 1 μg of RNA was primed with oligo (dT) using a reverse transcriptase kit from Promega according to the manufacturer’s instructions. TLR2mRNA-sense: 5′-CACCACTGCCC-GTAGATGAAG-3′, TLR2mRNA-antisense: 5′-AGGGTA-CAGTCGTCGAACTCT-3′; TLR5mRNA-sense: 5′-GCA-GGATCATGGCATGTCAAC-3′, TLR5mRNA-antisense: 5′-ATCTGGGTGAGGTTACAGCCT-3′; GAPDH-sense: 5′-AGGTCGGTGTGAACGGATTTG-3′, GAPDH-anti-sense: 5′-TGTAGACCATGTAGTTGAGGTCA-3′. The cDNA production was amplified in a qPCR instrument from Roche. The conditions for amplification were as follows: pre-denaturation for 30 s at 95°C for 1 cycle; denaturation for 10 s at 95°C, annealing for 30 s at 58°C and extension for 30 s at 72°C for a total of 40 cycles of PCR. Three replicates were prepared for each sample in PCR detection and data were analyzed using the 2–ΔΔCt method, where ΔCt = Ct target gene – Ct reference gene, and ΔΔCt = ΔCt test group – ΔCt control group.
Ethical approval and consent to participate
All animals were purchased from the Laboratory Animal Center of Academy of Military Medical Sciences (License: SCXK (Jing) 2016–0006) and housed in the Experimental Animal Service Center of the PLAGH. The animal use protocol had been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the PLAGH.
Statistical analysis
The data were expressed as means ± standard deviation (SD). Quantitative variables among groups were compared with one-way ANOVA, post-hoc test, and least significant difference (LSD) test. The correlation analysis between indicators was conducted using the Pearson or Spearman correlation test. A p-value < 0.05 was considered significant. All statistical analyses of the experimental data were performed with SPSS 19.0 software (Chicago, IL, United States).
Results
Assessment of general health
The animals in the SH group remained in good health throughout the experiment; they were lively with glossy fur, normal appetite and no narcolepsy. However, for those in the BDL group, the urine turned yellow 24 h after BDL, and skin and hair, especially ear tips and tails, turned yellow 48 h after BDL. Drowsiness and decreased mental state and appetite were commonly observed after BDL. The BDL mice ate markedly less food and there was a slowly increasing trend of body weight. Weight gain was more significant in SH mice compared with that in BDL mice. The segment of the common bile duct proximal to the ligature was found dilated at collection of specimens on the 3rd days after the operation in BDL mice models. Swelling and yellow staining of the liver were evident on the 5th day after the operation.
Liver function measurement
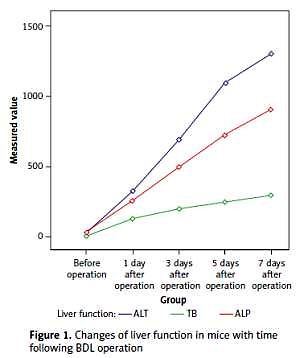
Serum levels of TB, ALT and ALP were increased several hours after BDL and the liver function deteriorated with time. The postoperative levels of TB, ALT, and ALP in the BDL group were compared with those of the SH group at each corresponding time point, and the differences were statistically significant (p < 0.01, Table I, Figure 1).
Table I
Serum ALT, TB and ALP levels of the two groups before and after operation (x ± s)
Histopathological changes
The intestinal villi are long and thin before the BDL operation. In BDL mice, there was blunting of intestinal villi. With the prolonged obstruction, a dilated subepithelial space with submucous edema-induced separated epithelia and lamina propria were observed. It was also noted that the continuity of the epithelium was destroyed and even some of the epithelium was missing, especially the obvious exfoliation and rupture of the apical epithelium. However, no obvious pathological change was observed in the SH group. The grade of mucosal injury increased gradually in the BDL group, which was significantly higher compared to that in the SH group on the 3rd, 5th and 7th days (p < 0.01, Table II, Figure 2).
Table II
Chiu grading of the two groups before and after operation (x ± s)
Figure 2
Histopathology of terminal ileum in the BDL group (200×; hematoxylin-eosin stain). A – Before operation, B – 1 day after operation, C – 3 days after operation, D – 5 days after operation, E – 7 days after operation, F – Chiu grading of intestinal mucosa at different time points in the BDL group. A–E – The intestinal villi are long and thin before the operation; blunted and sparsely messy villi and increased inflammatory cells as well as dilated subepithelial space with submucous edema-induced separated epithelia and lamina propria were observed with prolonged obstruction. It was also noted that the continuity of the epithelium was destroyed and even some of the epithelium was missing. F – The Chiu grading of intestinal mucosa increased gradually in the BDL group with the extension of OJ time
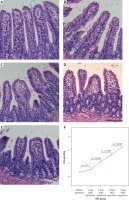
Quantitative detection of intestinal flora
Bacterial biomass of the three normal intestinal microflorae, including Bifidobacteria, Lactobacillus and Escherichia coli, was significantly changed in the BDL mouse model with time, indicating that dysbacteriosis developed in the OJ mice. The amounts of Bifidobacteria and Lactobacillus showed downward trends in the intestinal tract of the BDL group, and were significantly lower than those of the SH group on the 3rd, 5th and 7th days (p < 0.01). Furthermore, the amount of Escherichia coli was gradually increased in the intestinal tract of the BDL group, and it was significantly higher than that of the SH group on the 3rd, 5th and 7th days (p < 0.01) (Table III, Figure 3).
Table III
Intestinal flora of the two groups before and after operation (CFU/g, x ± s)
Figure 3
Changes in bacterial biomass of 3 intestinal microflorae with time after BDL. The amounts of Bifidobacteria and Lactobacillus showed downward trends in the intestinal tract of the BDL group. Furthermore, the amount of Escherichia coli was gradually increased in the intestinal tract of the BDL group
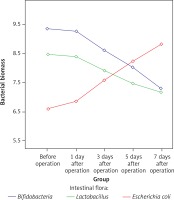
Immunohistochemistry for TLR2, TLR5 and NF-κB p65
TLR2 was weakly expressed on the surface of mucosa of the terminal ileum at different time points in the SH group, and the expression of TLR2 on the surface of mucosa of the terminal ileum in the BDL group was gradually increased with time after the operation. TLR2 was diffusely or granularly distributed in brown yellow color and highly expressed TLR2 was also observed in the inflammatory cells in the lamina propria near the epithelial cells. In addition, a low level of TLR5 was detected in the mucosa of the terminal ileum in the BDL group and the SH group and no significant changes in the staining intensity over BDL time were found. Weakly positive expression of NF-κBp65 protein was detected in the mucosa of the terminal ileum at different time points in the SH group, and cytoplasm in a small amount of cells was light yellow with the nucleus unstained, while cytoplasm in the cells of the BDL group showed enhanced brown yellow staining and a visibly stained nucleus and the staining intensity was increased over BDL time, which was significantly higher than that of the SH group (Figures 4–6).
Figure 4
Expression of TLR2 in the terminal ileum (200×; immunohistochemistry). A – TLR2 was weakly expressed on the surface of mucosa of the terminal ileum in the SH group, B – The expression of TLR2 on the surface of mucosa of the terminal ileum in the BDL group was obviously increased on the 7th day after the operation. TLR2 was diffusely or granularly distributed in brown yellow color and highly expressed TLR2 was also observed in the inflammatory cells in lamina propria near the epithelial cells, C – The expression of TLR2 increased gradually in the BDL group with the extension of OJ time
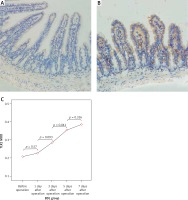
Figure 5
Expression of TLR5 in the terminal ileum (200×; immunohistochemistry). A–B – Low level of TLR5 was detected in the mucosa of the terminal ileum in the SH group and the BDL group on the 7th day after the operation and there were no significant changes in the staining intensity. C – The changes in the expression of TLR5 were not significant at each time point in the BDL group
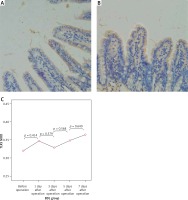
Figure 6
Expression of NF-κBp65 in the terminal ileum (.200; immunohistochemistry). A – Weakly positive expression of NF-κBp65 protein was detected in the mucosa of the terminal ileum in the SH group, and cytoplasm in a small amount of cells was light yellow with nucleus unstained, B – The cytoplasm in the cells of the BDL group showed enhanced brown yellow staining and visibly stained nucleus on the 7th day after the operation, C – The expression of NF-κBp65 increased gradually in the BDL group with the extension of OJ time
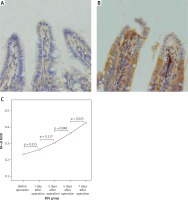
The expression levels of TLR2, TLR5 and NF-κBp65 proteins in the mucosa of the terminal ileum were determined by the Image Pro Plus 6.0 image analysis system. The positive expression area (Aera) and the integral optical density (IOD) were recorded and the mean integral optical density (MOD = IOD/Area) was calculated. A greater MOD value indicated stronger expression. MOD values of TLR2 in the BDL group at different time points (before operation, 1 day after operation, 3 days after operation, 5 days after operation, 7 days after operation) were 0.21 ±0.05, 0.23 ±0.06, 0.28 ±0.07, 0.35 ±0.10 and 0.39 ±0.09; MOD values of NF-κBp65 in the BDL group at different time points were 0.23 ±0.05, 0.26 ±0.06, 0.31 ±0.05, 0.36 ±0.06 and 0.43 ±0.08; both were increased gradually with the extension of OJ time. Also, there were significant differences when compared to those in the SH group on the 3rd, 5th and 7th days (TLR2 respectively p = 0.043, p = 0.017, p < 0.001; NF-κBp65 respectively p = 0.037, p = 0.006, p < 0.001). However, the expression of TLR5 showed no significant difference from the SH group at different time points in the BDL group (p > 0.05); the MOD values were 0.32 ±0.07, 0.35 ±0.07, 0.33 ±0.08, 0.35 ±0.06 and 0.36 ±0.09 (Figures 4–6).
Expression of TLR2 and TLR5 mRNA
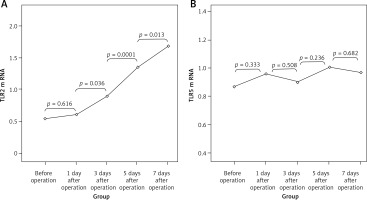
There were no significant differences in the expression of TLR2 and TLR5 mRNAs at different time points in the SH group (p > 0.05). TLR2 mRNA values (2–ΔΔCt) in the BDL group at different time points were 0.54 ±0.17, 0.61 ±0.18, 0.89 ±0.24, 1.35 ±0.38 and 1.69 ±0.41, which was increased gradually with the extension of OJ time. Also, there were significant differences when compared to those in the SH group on the 3rd, 5th and 7th days (respectively p = 0.032, p = 0.008, p < 0.001). However, the expression of TLR5 mRNA in the BDL group showed no significant difference from the SH group at different time points (p > 0.05); the values (2–ΔΔCt) were 0.87 ±0.18, 0.96 ±0.17, 0.90 ±0.21, 1.01 ±0.23 and 0.97 ±0.20 (Figure 7).
The relationship between the expression of TLR2 and the damage of intestinal mucosa and NF-κB in OJ
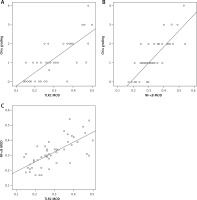
There were positive correlations between the grade of mucosal injury and expression of TLR2 (r = 0.767, p < 0.001) and NF-κB (r = 0.817, p < 0.001) in the BDL group. In addition, NF-κB expression was positively correlated with TLR2 expression (r = 0.607, p < 0.001) (Figure 8).
Discussion
The OJ is commonly seen in clinical practice. It is an intractable disorder because it is often complicated with multiple organ dysfunctions [17, 18]. The mechanism underlying the development of OJ has not yet been fully clarified. During OJ, intestinal mucosal barrier dysfunction and intestinal immune function decline might result in endotoxemia and intestinal bacterial translocation, which are important contributors to disease progression or death in patients [19–21]. Several reports describe spontaneous bacterial infections and sepsis in patients with OJ [22]. Under normal circumstances, the large number of bacteria in the intestinal tract cannot enter the body tissue or blood circulation due to the mechanical, biological, chemical and immunological barriers of the intestine. When the intestinal mechanical barrier is damaged, endotoxins enter the blood circulation, causing endotoxemia. Then, the intestinal bacteria can migrate into the intestinal lymph nodes, blood, liver or spleen, causing bacterial translocation [23–29]. Therefore, how to protect the intestinal barrier function as well as to reduce the intestinal permeability and subsequently to decrease bacterial translocation in OJ is the key to the effective reduction of morbidity and mortality of OJ.
Common BDL in animals is a popular method used to induce OJ [30]. Animals such as rats and rabbits were previously used to construct BDL models, which are characterized by prolonged modeling time and high cost. Besides, there is an absence of gallbladder structure in rats, which is different from the human biliary structure. In the present study, a mouse model was used due to the fact that mice have a gallbladder structure and share homologous genes with humans to a great extent, providing more advantages in studying the liver and the biliary tract diseases. It was observed that serum levels of TB, ALT and ALP were increased in the BDL group several hours after the operation. Meanwhile, the liver function deteriorated with the extension of the obstruction time. Moreover, obvious dilatation of the common bile duct adjacent to the ligature site and obvious swelling and yellowing of the liver were also observed 3 to 5 days after the operation, suggesting that the model was successfully established. Meanwhile, pathological damage of intestinal mucosa was also aggravated with the prolonged biliary obstruction time. We observed that mice with OJ exhibit circular muscularis thinning and decreased mucosal thickness and villous height. It was also observed with the inflammation and edema under the epithelium, damaged continuity of epithelium, and even the absence of partial mucosal epithelium. These changes have a crucial role in OJ because they promote enterogenous endotoxemia and bacterial translocation. After entering the blood, endotoxin can stimulate the opening of calcium channels in cell membranes of monocytes, macrophages, granulocytes and endothelial cells, and cause calcium influx, which leads to the production of cytokines such as tumor necrosis factor, interleukin, oxygen free radicals, prostaglandin and platelet activator that induce immunosuppression and aggravate gastrointestinal mucosal injury to even acute renal failure, thus forming a vicious circle [31–33]. This is also the cause of infections, gastrointestinal bleeding, renal failure, even multiple organ failure and other complications often occurring in patients with OJ after surgery.
TLRs are one of the cell membrane receptors of the innate immune system and the pathogen pattern recognition receptors that can recognize the pathogen associated molecular patterns (PAMPs) conserved in many pathogenic microorganisms. TLRs are also expressed in various cells in the intestinal mucosa and mainly involved in product identification and inflammatory signal transduction in pathogenic microorganisms, serving as a bridge between innate and acquired immunity [34]. Currently 12 TLRs are found in humans and 11 TLRs in mice, and different TLRs can specifically recognize various PAMPs on microbes [35]. TLR2 is a “pattern” recognition receptor with a broad spectrum of recognition capacity. It recognizes the cell wall components of many bacteria including the gram positive bacteria, gram negative bacteria, fungi and spirochetes, and plays an important role in acute and chronic infections caused by a variety of microorganisms [36]. Studies in recent years found that the expression of TLR2 in intestinal mucosa of ulcerative colitis (UC) patients is significantly increased compared with that of non-UC patients [37], indicating that TLR2 and its signal transduction pathway play an important role in intestinal diseases. TLR5 is a receptor in the immune system that recognizes flagellin from bacteria. After binding to flagellin, TLR5 mediates the innate immune response and inflammatory response against pathogens [38]. A study by Sánchez-Munoz et al. [39] showed that TLR5 is highly expressed in epithelial cells of the intestinal mucosa in UC patients and positively related to the severity of the disease and the expression of inflammatory factors (IL-6 and TNF). In the present study, it was found that TLR2 and TLR5 were slightly or weakly expressed on the mucosal surface of the terminal ileum in normal mice, which may be closely related to the immune tolerance of the intestines. Normal intestinal mucosa can tolerate sustained stimulation from TLR ligands such as LPS and poorly respond to the external environment to maintain a basic level of activation [40–45]. This can be explained by that the low level of TLR expression reduces its contact and recognition probability for PAMPs such as intestinal commensal bacteria, pathogenic bacteria or other food antigens to produce an appropriate immune response and maintain the intestinal mucosal balance, namely, intestinal mucosa homeostasis. Meanwhile, the precise negative regulation mechanism in the intestinal mucosa (extracellular, transmembrane and intracellular negative regulators) weakens the activation of TLRs and avoids the excessive intestinal mucosal immune response induced by TLRs. In the case of OJ, whether the intestinal TLRs will be activated to participate in the destruction of the intestinal mucosal barrier has not yet been reported. In the present study, the results of immunohistochemistry and PCR confirmed that expression levels of TLR2 protein and mRNA in intestinal mucosa were both significantly increased in BDL mice compared with those of the SH group and the expression intensity was positively correlated with the severity of pathological intestinal mucosal injury (r = 0.767, p < 0.001). It was speculated that overexpression of TLR2 was involved in the occurrence and development of intestinal mucosal injury in mice with OJ. Interestingly, expression of TLR5 in the terminal ileum at each time point after BDL modeling was not significantly changed compared with the SH group. Possibly TLR5 was not involved in the pathogenesis of intestinal mucosal injury during OJ, or 7 days after BDL was not long enough to observe the changes in TLR5.
The mechanism of the up-regulated TLR2 expression in the terminal ileum in OJ is not established and we speculated that dysbacteria may be one of the main causes. There are more than 500 kinds of intestinal bacteria, and obligate anaerobes, such as Bifidobacterium and Lactobacillus, are the dominant intestinal species under physiological conditions, which mainly reside in the colon and distal small intestine and account for more than 99% of the total intestinal microflora. The remaining 1% are mainly composed of conditional pathogenic bacteria including Escherichia coli, bacilli, and Enterococcus, and transient microflora, the reproduction of which is inhibited by the dominant counterparts in a dynamic balance [46]. Our present study also proved that the main intestinal microflora in normal mice included Bifidobacterium and Lactobacillus and the amount of Escherichia coli was relatively minor. However, the amount of Bifidobacterium and Lactobacillus in the intestinal tract gradually decreased with the prolongation of obstruction time while that of Escherichia coli gradually increased, resulting in intestinal microflora imbalance. One of the possible reasons is that bile salt, the effective component of bile, fails to pass through the intestinal tract and exert the antibacterial and “cleaning agent” function during OJ [47], causing a large number of enteric pathogens. Research has confirmed that the reproduction of dominant bacteria is significantly inhibited once the intestinal microflora is unbalanced, and that of conditional and transient pathogenic bacteria is significantly enhanced. Consequently, a series of downstream pathological changes occur, resulting in inflammatory injury of the intestinal mucosa [48, 49]. We speculated that epithelial cells in the intestinal mucosa and inflammatory cells in the lamina propria directly expose to massive intestinal microflora once the intestinal mucosal barrier is damaged, and the immune tolerance of the host intestinal mucosa against the intestinal microflora is compromised. TLR2 expression in intestinal epithelial cells and lamina propria cells was then highly induced; it binds to its corresponding ligand binding, to activate the downstream signal transduction pathways and enhance the expression of various proinflammatory factors. These proinflammatory cytokines may also further enhance the expression of TLR2. The present study also suggested that the most significant intestinal microflora imbalance and the peak TLR2 expression were both observed on day 7 after BDL and the concurrence indicated that the imbalance of intestinal microflora may be one of the reasons for the abnormal high expression of TLR2 in intestinal mucosa during OJ.
NF-κB is an important transcription factor that widely exists in the body to regulate the expression of various inflammation- and immune-related genes. The main form of NF-κB is dimer P65/P50 with a transcription activity binding site only in the C-terminal of p65 [50]. So the amount of activated NF-κB can be determined by directly measuring NF-κBp65. NF-κBp65 is activated and translocated into the nucleus, where it binds to the κB motif in the promoter or enhancer of the target genes, to induce the transcription of many factors [51]. The expression of most inflammatory mediators, such as IL-1, TNF-α, NO, IL-8, and E-selectin, is regulated by NF-κB during the inflammatory response [52]. Studies [53] have shown that TNF-α level in OJ is significantly increased, which is related to the activation of NF-κB. The increased TLR2 expression can activate the Toll-like receptor signaling pathway, the TLR2-IRAK-NF-κB pathway, and induce the high activation of NF-κB. This is also confirmed in our present study, i.e., the expression of NF-κBp65 was increased gradually with the extension of OJ time, which was positively correlated with the expression of TLR2 (r = 0.607, p < 0.001) and the severity of pathological damage of intestinal mucosa (r = 0.817, p < 0.001). Immunohistochemistry showed that the expression and distribution of NF-κBp65 were comparable to those of TLR2. NF-κBp65 was expressed in both cytoplasm and nucleus, with stronger expression in the nuclei vs. the cytoplasm sometimes, suggesting that NF-κB was activated during OJ. Notably it was found in our study that the increase of TLR2 mRNA and protein level was significantly reduced 5 days after BDL. This tendency was not observed in NF-κBp65, suggesting that other TLRs may also be involved in the activation of NF-κB during OJ. Under normal circumstances, a certain amount of NF-κB plays a crucial role in maintaining intestinal mucosal integrity and coordinating the innate immunity and adaptive immunity in antimicrobial activities. However, continuously high expression of NF-κB may lead to excessive release of inflammatory factors and a severe inflammatory response, which aggravates the intestinal mucosal barrier destruction and starts up a vicious circle. Some experimental studies showed that NF-κB may induce or aggravate small intestinal ischemia/reperfusion injury [54], promote intestinal epithelial cell apoptosis [55], and thereby induce intestinal damage.
In conclusion, the intestinal environment is destroyed and dysbacteriosis occurs in the BDL-induced OJ mouse model. Tolerance of intestinal mucosa to the environment is unbalanced when facing dangerous stimuli such as gram positive and negative bacteria, leading to the high expression and activation of TLR2 in intestinal mucosa. Activated TLR2 induces activation of NF-κB via the TLRs/NF-κB signaling pathway and further enhances the release of various inflammatory mediators, thus aggravating the damage of intestinal mucosa and bacterial translocation, and leading to severe complications. Therefore, effectively preventing flora imbalance and blocking the over-expression and activation of the small intestinal TLR2 pathway may play an important role in reducing the occurrence and development of intestinal mucosal barrier damage and systemic complications during OJ. Moreover, it should be noted that there are still some limitations of this study. 1) The established BDL models cannot fully represent the pathophysiological status of OJ, especially chronic OJ common in clinic and some special types of OJ. Further, these models are always expensive and complicated in operation. Thus, it is difficult to obtain a large sample size and stable models. 2) Some studies have shown that TLRs may be involved in the occurrence and development of liver injury and liver fibrosis during OJ [56–59]. The interaction between the liver and the gut complicates the role of TLRs in the pathogenesis of OJ. Therefore, further in-depth and comprehensive research is needed to lay a solid foundation for the effective treatment of OJ in the clinic.