Introduction
Helicobacter pylori infection is still an important worldwide medical problem. Despite progress in the diagnostics and therapy, in various regions of the world it affects more than half of the population. In the majority of cases (about 80%) it is asymptomatic, but discrepancies in the assessment result from the differences in the diagnostic procedures. In population studies, serological methods and determination of the level of IgA/IgG [1–4] or IgM antibodies are used most commonly [5], whereas monoclonal stool antigen tests and PCR methods are applied less frequently [6–8]. However, the urea breath test – 13C-UBT – has been recognized to be sufficiently accurate and simple to use in screening of patients [9–11].
Long-term clinical observations indicate that this is not an innocent infection. Its possible consequences include malabsorption of iron and vitamins, secretory dysfunction of the pancreas and intestinal myoelectric activity disorders [12–16]. Moreover, it leads to destructive changes in the gastric and duodenal mucosa and can be the cause of asymptomatic peptic ulcers [17–19]. In asymptomatic infections eradication of H. pylori is a preventive management of gastric cancer, especially in patients with a family history of this disease [20–22].
Another issue is to explain why H. pylori infection induces clinical symptoms in only 15–20% of subjects. It was believed to depend on the virulence of H. pylori strains, but in many studies conducted in patients with symptomatic and asymptomatic infection, there were no significant differences in the prevalence of virulent CagA and VacA strains [23–28], although there is still a search for them [29, 30]. Moreover, no morphological differences have been found in gastric mucosa or in the level of pro-inflammatory cytokines [31–33].
However, it has been indicated that impaired oxygen metabolism is observed in asymptomatic infection [34, 35], and the presence or absence of signs of infection depends on many factors, including epigenetic ones – complex interactions between bacteria and the host and environmental factors [36, 37]. Cigarette smoking and the use of nonsteroidal anti-inflammatory drugs can cause inflammation exacerbation [38–40]. There are no consistent views on the impact of dietary components and alcohol on this process [41, 42].
In general, it can be concluded that external gastrotoxic factors or deficiency of enteroprotective factors may adversely affect the natural history of chronic gastritis in patients infected with H. pylori.
From among gastroprotective agents melatonin plays an important role [43]. In dyspeptic patients, particularly with epigastric pain syndrome, its synthesis in gastric mucosa and serum level are decreased [44–47]. The role of serotonin in the pathogenesis of dyspepsia is less known. Patients with dyspepsia without H. pylori infection had elevated serum serotonin levels and increased urinary excretion of its metabolite (5-HIAA) [48, 49]. However, the concentration of serotonin at the site of its synthesis is more important, where as a tissue hormone it exhibits high physiological activity. In the gastrointestinal tract the main source of serotonin is enterochromaffin cells in which the exogenous L-tryptophan is converted to serotonin involving tryptophan hydroxylase (TpH-1) [50]. Serotonin secretion is stimulated by various factors, including the cholinergic and adrenergic system, nutrients, chemical compounds, bacterial toxins and others [51]. The effect of H. pylori infection on this process is not understood.
The aim of the study is to evaluate the expression of tryptophan hydroxylase in gastric mucosa of patients with symptomatic and asymptomatic H. pylori infection in relation to the intensity of bacterial colonization and severity of dyspeptic symptoms.
Material and methods
Patients
The study included 90 subjects (61 women and 29 men), aged 35–49 years, divided into three groups:
group I (control, n = 30) – healthy volunteers without H. pylori infection and without gastrointestinal tract pathology;
group II (n = 30) – subjects with asymptomatic H. pylori infection;
group III (n = 30) – H. pylori-infected patients with dyspeptic symptoms, mainly fasting and nocturnal epigastric pain.
All patients underwent endoscopic examination of the upper gastrointestinal tract and histological assessment was performed using hematoxylin-eosin and Giemsa staining. In order to confirm H. pylori infection the urea breath test (13C-UBT) was applied.
Inclusion criteria: superficial infection of gastric mucosa (grades 1 and 2 according to the Sydney Classification [52], the presence of bacteria in preparations stained by Giemsa, the lambda ratio (Δ) – more than 4‰ 30 min after the marker ingestion, using the urease breath test on a FANCI-2 system (Fisher Instruments, Germany), symptoms of minimum 6-month duration and no improvement after antacid or prokinetic drugs.
Exclusion criteria: other organic, metabolic and mental diseases, previous abdominal surgery, receiving any medication, smoking, alcohol abuse.
Study design and procedures
The severity of dyspeptic symptoms was estimated with the Visual Analog Scale (VAS; 1–10 points).
Laboratory tests included blood cell count, C-reactive protein, glycosylated hemoglobin, bilirubin, alanine and aspartate aminotransferase, γ-glutamyl transpeptidase, alkaline phosphatase, amylase, lipase, urea, creatinine, cholesterol (HDL and LDL), and triglycerides.
Seven days prior to the evaluations, all medications were withdrawn and the same diet was used by all subjects with a similar daily amount of products rich in L-tryptophan.
Patients with a symptomatic H. pylori infection were subjected to 14-day antibacterial treatment with: pantoprazole (2 × 40 mg), amoxicillin (2 × 1000 mg), levofloxacin (2 × 500 mg). The follow-up 13C-UBT test and control endoscopy with biopsy of the same parts of the stomach were performed 12 weeks after the end of the treatment.
Material for histological and molecular examinations was collected from the antral part (4 samples) and the upper part of the gastric body (4 samples).
The level of mRNA was estimated with RT-PCR and qPCR and, for this purpose, 50 mg of gastric tissue was used. Gastric tissue was excised and placed immediately in RNAlater reagent (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. For processing, samples were removed from RNAlater and the RNA pellet was reconstituted in 100 µl of RNase- and DNase-free water and processed with the RNeasy Mini Kit (Qiagen) according to the manufacturer’s RNA Cleanup protocol with DNase I treatment (Qiagen). Purified RNA samples were stored at –20°C until use. The quantity and quality of RNA were evaluated spectrophotometrically. The cDNA synthesis was performed with oligo (dT) using the ImProm-II Reverse Transcription System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The qPCR was conducted with PCR Master Mix (Promega) and specific primers to amplify fragments of the cDNA of the tryptophan hydroxylase (TpH-1) genes. The qPCR products were separated on an 8% polyacrylamide gel stained with ethidium bromide. The products were then subjected to densitometry to determine the level of analyzed genes’ mRNA. Expression of the housekeeping hypoxanthine phosphoribosyltransferase (HPRT) gene was used to normalize the level of TpH-1 expression.
Statistical analysis
The non-parametric Kruskal-Wallis test was used for the comparison of TpH-1 expression levels and results of 13C-UBT. The Mann-Whitney U test was applied for median comparison. The correlation between the above parameters and intensity of H. pylori infection and severity of epigastric pain was assessed by Pearson’s correlation coefficient and the linear regression equation. Excel 2007 and Statistica 9.1 (license no. AXAP 106E7359M-F) software were used for statistical analysis.
Results
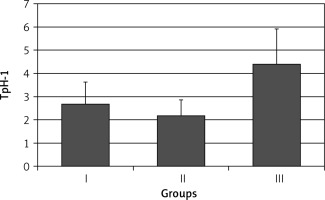
Compared to healthy subjects (group I) TpH-1 expression in antral mucosa in patients with asymptomatic H. pylori infection was similar – 2.69 ±0.97 and 2.28 ±0.69, respectively (p > 0.05).
Higher expression of TpH-1 in this part of the stomach was found in patients with symptomatic infection (group III): 4.40 ±1.64 (p < 0.001) (Figure 1).
Figure 1
Expression of tryptophan hydroxylase (TpH-1) in antral gastric mucosa in healthy volunteers (group I), subjects with asymptomatic (group II) and those with symptomatic (group III) Helicobacter pylori infection. Statistical differences: I vs. II p > 0.05; I vs. III p < 0.001; II vs. III p < 0.001
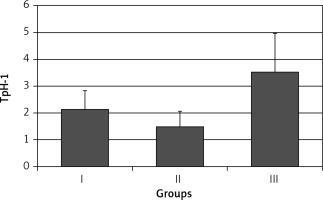
The expression of TpH-1 in the gastric body mucosa was: 2.16 ±0.70 in group I, 1.57 ±0.52 in group II (p > 0.05), and 3.40 ±1.51 in group III (p < 0.001) (Figure 2).
Figure 2
Expression of tryptophan hydroxylase (TpH-1) in body gastric mucosa in healthy volunteers (group I), subjects with asymptomatic (group II) and those with symptomatic (group III) Helicobacter pylori infection. Statistical differences: I vs. II p > 0.05; I vs. III p < 0.001; II vs. III p < 0.001
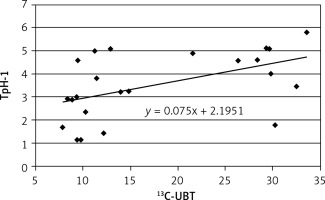
A positive correlation was found between results of 13C-UBT and TpH-1 expression in antral (r = 0.575, Figure 3) and less in gastric body mucosa (r = 0.499, Figure 4) in patients with symptomatic infection (group III), probably caused by different intensity of H. pylori colonization.
Figure 3
Correlation between intensity of Helicobacter pylori infection (13C-UBT) and expression of tryptophan hydroxylase (TpH-1) in antral gastric mucosa; r = 0.575, p < 0.05
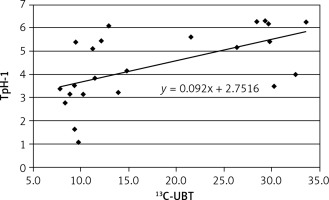
Figure 4
Correlation between intensity of Helicobacter pylori infection (13C-UBT) and expression of tryptophan hydroxylase (TpH-1) in body gastric mucosa; r = 0.4993, p > 0.05
In group II, the correlation between the results of 13C-UBT and TpH-1 expression in antral and gastric body mucosa was lower (r = 0.529, r = 0.455, respectively).
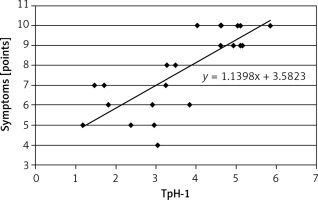
A positive correlation was observed between TpH-1 expression in antral (r = 0.855; Figure 5) and gastric body mucosa and the intensity of dyspeptic symptoms (r = 0.802; Figure 6).
Figure 5
Correlation between expression of tryptophan hydroxylase (TpH-1) in antral gastric mucosa and severity of dyspeptic symptoms; r = 0.8549, p < 0.01
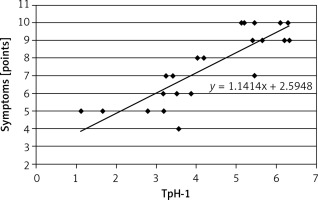
Figure 6
Correlation between expression of tryptophan hydroxylase (TpH-1) in antral gastric mucosa and severity of dyspeptic symptoms; r = 0.8019, p < 0.001
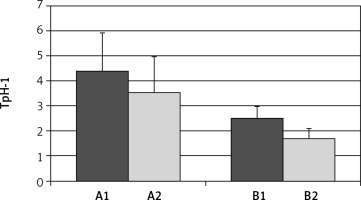
Eradication of H. pylori was obtained in 23 patients (76.6%). In these patients the expression of TpH-1 decreased from 4.40 ±1.64 to 2.51 ±0.47 (p < 0.001) in antral mucosa and from 2.28 ±0.69 to 1.69 ±0.39 in gastric body mucosa (p < 0.05) (Figure 7).
Figure 7
Expression of tryptophan hydroxylase (TpH-1) in antral (A) and body (B) gastric mucosa, before (A1, B1) and after (A2, B2) eradication of Helicobacter pylori. Differences in both parts of gaster are statistically significant; A1 vs. A2 p < 0.01; B1 vs. B2 p < 0.001
After 3 months dyspeptic symptoms were absent in 9 (39.1%) patients, decreased to a varying degree in 8 (34.8%) patients, and did not change in 6 (26.1%) patients.
Discussion
Serotonin contents in the alimentary tract constitute approximately 90% of the total body pool of this hormone. In normal conditions it is secreted along the whole tract, mainly by enterochromaffin cells [53–56]. However, in inflammatory foci other cells may also be its source [57–59].
The effects of serotonin on the gastrointestinal tract depend on the type of the stimulated receptor. So far seven classes of serotonin receptors have been identified – from 5-HT1 to 5-HT7 – within which several subtypes can be distinguished [60].
Gastric motility is stimulated by activation of 5-HT2, 5-HT3 and 5-HT4 receptors, but it is inhibited by the 5-HT1 receptor. Stimulation of the 5-HT1A receptor causes fundus relaxation, whereas 5-HT1p receptors are involved in the initiation of peristalsis and gastric secretion [61, 62].
5-HT3 receptors are located in the gastrointestinal tract, mainly on vagal sensory fibers which convey stimuli from the gastrointestinal tract to the central nervous system. Activation of 5-HT3 is responsible for the occurrence of visceral hypersensitivity, early satiety, nausea and vomiting [63, 64]. Serotonin plays a special role in the mechanisms of visceral sensation. Nerve impulses arising within the gastrointestinal tract after reaching the brain are transmitted to the limbic system – which is responsible for the motivational-affective component of visceral pain, such as anxiety, suffering, and emotions – and to the sensory cortex, where pain is located and consciously perceived [65, 66].
The mechanism of the onset of dyspeptic symptoms in H. pylori-infected subjects is complex and still unclear. The pain usually occurs upon fasting and at night but often also after meals, and it is not accompanied by early satiety or food retention. Studies to date have not demonstrated in these cases adverse effects of serotonin on gastric motility and secretion [67–69]. Factors that induce visceral pain include, among others, mechanical stimuli and inflammatory mediators (cytokines, prostaglandins). Serotonin with the main participation of 5-HT3 receptors plays a role as a neurotransmitter in transmitting visceral sensation, regardless of the nature of excitatory stimuli [70, 71].
Helicobacter pylori infection causes chronic gastritis with different activity. Inflammatory and destructive changes of gastric mucosa are the result of the adverse effect of bacterial cytotoxins and increased secretion of pro-inflammatory cytokines [72–74]. Eradication of bacteria results in lower levels of cytokines [75] but it does not always relieve dyspeptic symptoms. Likewise, it is not known why H. pylori colonization triggers dyspeptic symptoms only in some infected subjects. Precise knowledge of serotonin synthesis and metabolism may be the key to explain these differences. Serotonin is an important mediator of inflammation, promotes the chemotaxis of granulocytes, lymphocytes, macrophages to inflammatory foci and enhances the secretion of pro-inflammatory cytokines [76, 77].
The results of our study indicate that the expression of tryptophan hydroxylase in the gastric mucosa with symptomatic H. pylori infection is significantly higher than in patients without symptoms. It may indicate the direct induction of TpH-1 by pro-inflammatory cytokines. Cytokines may also cause proliferation of different cells, including enterochromaffin cells. An increased number of them was found previously in H. pylori-infected patients [53].
Downregulation of TpH-1 after H. pylori eradication indicates the role of the bacteria in the disturbances of serotonin homeostasis in the stomach. However, the level of expression of tryptophan hydroxylase may depend on many factors, including the number of enterochromaffin cells [78], TpH-1 gene polymorphism [79] or the presence of antibodies directed against this enzyme [80].
Regardless of the mechanism, the increase in the level of serotonin can be the cause of visceral pain in various parts of the gastrointestinal tract. It was observed, among other things, that hyperserotoninemia occurring in the appendix was manifested by chronic pain in the lower right abdomen [81]. The effect may vary depending on the density, type and structure of serotonin receptors.
Apart from H. pylori eradication the agents that inhibit gastric secretion are used in the treatment of pain in functional dyspepsia. When improvement is not observed, agents modulating the serotonergic system, including psychotropic drugs, are used. Good results were obtained when applying low doses of tricyclic antidepressants [82]. In recent years, serotonin reuptake inhibitors have been used but no consistent results have been obtained. Together with good evaluation [83, 84], there predominate conclusions that these drugs are not more effective than placebo [85, 86]. Their effect is to increase the concentration of serotonin in the gastrointestinal tract, which in the first phase of the treatment may increase abdominal pain and induce nausea and diarrhea. The risk of gastrointestinal bleeding related to platelet dysfunction is a limitation in the use of SSRIs [87–89]. On the other hand, these drugs are thought to possess antioxidant, anti-inflammatory and gastroprotective properties [90–92].
Assessment of the effectiveness of drugs that modulate the activity of the serotonergic system is difficult because they depend on both the effect on the structure of the gastrointestinal tract and on the CNS [93, 94]. Chronic dyspepsia is often an exponent of somatization of the depression and the use of SSRIs is justified, but their application requires careful analysis of the patient’s health. This group of drugs is heterogeneous, and the majority of them, besides inhibiting serotonin reuptake, exhibit agonist/antagonist activity in relation to various serotonin receptors.
In view of the above information, a new class of drugs selectively inhibiting TpH-1 is the hope for successful treatment of certain forms of dyspepsia and other disorders of the gastrointestinal tract [95, 96]. The results of current, mainly experimental studies are optimistic, but require extended clinical trials.
In conclusion, this study demonstrates that increased expression of TpH-1 in gastric mucosa plays an important role in pathogenesis of chronic dyspepsia.


