Introduction
Birt-Hogg-Dubé syndrome (BHDS) is a disease caused by autosomal dominant mutation in a folliculin gene (FLCN). The disease manifests by skin lesions (fibrofolliculomas, trichodiscomas, perifollicular fibromas, and acrochordons), lung cysts inducing recurrent pneumothoraces, and kidney neoplasms. BHDS was first described in 1977, and later Chung et al. associated the syndrome with lung cysts [1].
FLCN gene is located on a short arm of the chromosome 17 locus p12-q11.2; it belongs to the family of tumour suppressor genes [2]. The folliculin defect results in the malfunctioning of cell adhesion mechanisms as well as intercellular junctions [3]. In case of developing pulmonary abnormalities, there are suggestions that the “stretching mechanism”, which is most prominent in supradiaphragmatic regions, plays an important role. It results in the development of lung cysts located mostly in lower and middle lung zones. In tissue samples acquired from patients with BHDS small nonspecific air spaces are present; they represent cysts that are lined with alveolar epithelium. In 30% of patients, lung cysts may be the only BHDS manifestation.
Case 1
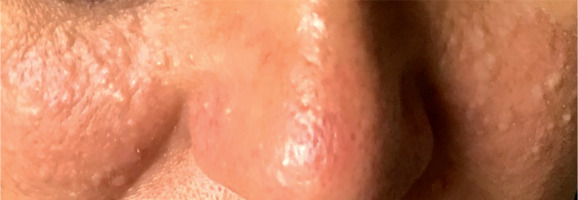
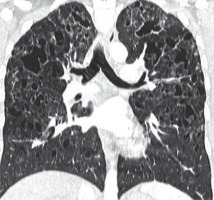
A 52-year-old woman presented to the outpatient pulmonology department with results of computed tomography (CT) examinations showing lung cysts located below the tracheal carina. The patient’s history was negative for smoking or exposure to any kind of pneumotoxic substance. The patient reported cough with sputum production lasting for a few years. Numerous, soft, whitish-yellow lesions were found on patient’s face during physical examination (Figure 1). They represented fibrofolliculomas, which, according to the patient, started to present when she was about 30 years old. Subsequent CT examination revealed multiple, thin walled, differently shaped cysts in both lungs, located predominantly in the lower lung zones (Figure 2). The largest cyst had dimensions of 11 × 10 × 3.1 cm (Figures 2B, 3). Abdominal ultrasound examination was normal. Ventilation parameters and diffusing capacity of carbon monoxide were within normal limits. In blood tests none of the inflammatory or cancer markers was elevated. During history gathering, the patient stated that her sister had similar lung cysts and that she had positive history of numerous recurrent pneumothoraces. Because of suspicion of BHDS, a gene test for FLCN was performed. The results were positive for c.1285delC mutation in one of the FLCN alleles, which confirmed the diagnosis of Birt-Hogg-Dubé syndrome.
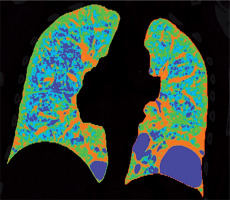
Figure 2
Baseline chest computed tomography, lung window: A) axial plane, B) coronal reconstruction. It shows multiple well-circumscribed, thin-walled, and variable sized lung cysts with round, oval, and irregular shapes with predominantly basal distribution. The largest cyst is located in the left lower lobe
Case 2
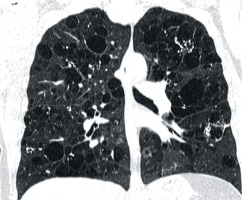
A 44-year-old woman, a sister of the aforementioned patient, whose history was also negative for smoking and exposure to other pneumotoxic substances, was hospitalised a few times because of recurrent pneumothoraces. Chest CT revealed numerous thin-walled lung cysts located mostly in lower lung zones (Figure 4A). The largest lung cyst had dimensions of 4.2 × 2.4 × 2.1 cm (Figures 4B, 5). The first pneumothorax occurred when the patient was 34 years old. After reappearance of the pneumothorax three years later, wedge resection of the right lung containing emphysematous bulle was performed (Figure 6). Simultaneously, mechanical abrasion of the pleural cavity was performed. A third pneumothorax occurred seven years later, during infection with persistent cough; it resolved spontaneously. During winters the patient reported dry cough and mild exertional dyspnoea. The patient had no skin lesions. Ventilation parameters and the diffusion capacity of carbon monoxide was within normal limits. Abdominal ultrasound examination was normal.
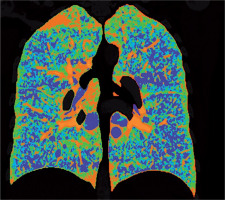
Figure 4
Baseline chest computed tomography, lung window: A) axial and B) coronal plane. Computed tomography scan shows characteristic distribution of lung cysts predominantly in medial aspect of middle and lower lung zones
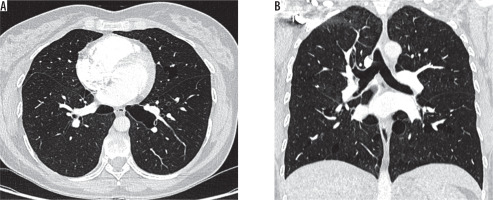
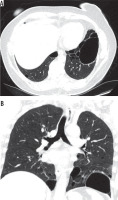
Figure 6
Chest computed tomography, lung window: A) axial plane, B) coronal plane. Recurrent pneumothoraces. There is a right-sided pneumothorax and small cysts in both lungs
In histopathological examinations of tissue samples acquired during wedge resection of emphysematous bullae, focal fibrosis and pleural hyperperfusion was noted.
Family history: The parents of the sisters are deceased, and presumably did not have any kind of skin, lung, or kidney lesions; however, we do not have access to their medical records. It should be suspected that one of the parents had congenital genetical mutation, which in turn passed to one of the children.
The presented patients also have a third sister whose high-resolution computer tomography (HRCT) was normal.
A 15-year-old daughter of the second patient has Raynaud syndrome but lacks any other symptoms; we are planning to perform gene test for mutations in FLCN gene after she reaches adulthood.
Discussion
Birt-Hogg-Dubé syndrome is a rare and hard to diagnose disease. In the whole world there are about 600 families with BHDS. In Poland it was diagnosed and published for the first time in 2016 [4]. Lung cysts start to occur when patients are 30-40 years old [5]. There is no correlation with patients’ sex or history of smoking. Lung cysts are found in 77-89% of patients, and pneumothoraxes develop in 33-38% [6].
Chest radiographs typically do not show any abnormalities. Sometimes poorly visible lucencies representing lung cysts or pneumothorax can be seen. CT examinations show cysts of variable shapes (round, oval, or lenticular), with well-defined walls, located mostly in middle and lower lung zones. Tobino et al. analysed computed topographies of patients with BHDS and found that most cysts were located in basal medial regions (58%); less frequently they were seen in basal peripheral regions (27%). The number of cysts varies from tens to hundreds, their size also varies greatly, but most have a diameter smaller than 1 cm, and approximately 40% adhere to pleura [7].
The most common clinical manifestation of BHDS are recurrent pneumothoraces.
Zbar et al. reported that the frequency of pneumothorax occurrence increases with the patient’s age [8]. The mean age of patients at the time of first pneumothorax occurrence was 38 years, and 75% of pneumothoraces are recurrent. There is a theory that the size and volume of lung cysts have positive correlation with pneumothorax occurrence risk. In the patients described in our article, pneumothoraces occurred only in one of the sisters; surprisingly, out of the two she had smaller cysts.
High-resolution computed tomography plays a crucial role in diagnosing BHDS. In differential diagnosis one should include other cystic lung diseases such as: emphysema, lymphangioleiomyomatosis (LAM), pulmonary Langerhans cell histiocytosis (PLCH), lymphoid interstitial pneumonia (LIP), and amyloidosis (Table 1) [9].
Table 1
Differential diagnosis of Birt-Hogg-Dubé syndrome (BHDS)
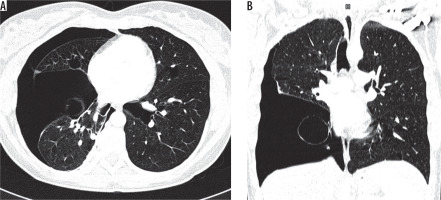
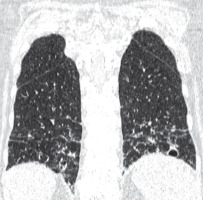
Patients with emphysema have a history positive for smoking, and bullae are usually located in upper lung zones, in contrast to BHDS in which they are generally located below tracheal carina (Figure 7). The greatest difficulty is the differentiation with LAM, especially coexisting with tuberous sclerosis (TSC). In both TSC and BHDS, skin lesions may be present, but in the case of TSC they take the form of: haemangioma, fibromas of the head, periungual fibromas, gingival fibromas, and shagreen patch. In BHDS lung cysts are round, lenticular, or oval shaped, in contrast to round shaped LAM cysts. Most of the lung cysts in BHDS are smaller than 10 mm; however, they can be as big as several centimetres in diameter, as in the presented cases. In patients with LAM the diameter of cysts ranges from 2 to 30 mm. Furthermore, cysts in LAM are evenly distributed throughout both lungs in contrast to BHDS where they are located in the middle and lower lung zones (Figure 8). Moreover, in BHDS cysts frequently adhere to pleura. BHDS occurs with equal frequency in women and men, LAM is a female disease, and the individual cases of the men described relate to patients with TSC. In patients with BHDS, pneumothorax is less common than in patients with LAM, but the tendency to relapse is similar to that in LAM. In about 80% of patients with TSC/LAM and about 50% of patients with sporadic LAM, kidney angiomyolipomas are found, while only about 30% of patients with BHDS have kidney lesions, both benign and malignant [10]. MRI of the head is useful in differentiating BHDS and TSC. In head MRI of TSC patients one can see cortical/subcortical glioneuronal tubers, subependymal glial nodules (SENs), and subependymal giant cell astrocytomas (SEGAs).
Figure 7
Pulmonary emphysema. Chest computed tomography, lung window, coronal reconstruction, showing severe emphysema in both lungs
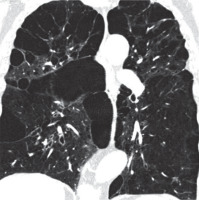
Figure 8
Lymphangioleiomyomatosis, chest computed tomography, lung window, coronal plane showing diffuse lung cysts with regular walls
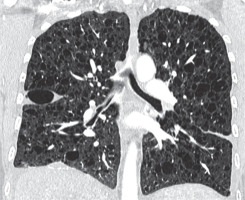
In CT examination of patients with pulmonary form Langerhans cell histiocytosis (occurring almost exclusively in smokers), in addition to bizarrely shaped (sometimes clover leave-like) lung cysts, centrilobular nodules are visible. In PLCH lesions localise in the upper and middle lung fields, saving diaphragmatic angles (Figure 9). The decisive test in these cases is the histological examination of lung samples, in which granulomas consisting of Langerhans cells showing expression of CD1a and langerin antigens are visualised [11]. In contrast, in patients with BHDS, only features of emphysema are detected in histological examination, as in the second presented case.
Figure 9
Langerhans cell histiocytosis. Baseline chest computed tomography, lung window, coronal plane demonstrates that the cysts are located predominantly in the upper and middle lung zones with sparing of costophrenic angle
Another disease that should be included in differential diagnosis is lymphoid interstitial pneumonia (LIP). Lymphoid interstitial pneumonia belongs to the group of rare interstitial pneumonias and is characterised by the presence of interstitial lung polyclonal lymphocytic infiltrates. In most cases, LIP is associated with autoimmune conditions, especially connective tissue disease (Sjögren’s syndrome); however, in rare cases it is an idiopathic disease. The HRCT shows lung cysts of various sizes with clearly defined walls, dominating in the lower lung fields. In addition, there are visible foci of centrilobular nodules, foci of ground glass opacities, thickening of inter-lobular septa, bronchiectasis, and recurrent pneumothoraces. There is a right pneumothorax with small cysts in both lungs (Figure 10).
Figure 10
Lymphoid interstitial pneumonia, chest computed tomography, lung window, coronal plane shows thin-walled cysts in a background of diffuse ground-glass opacity in the lower lung zones
Cysts are usually less numerous than in LAM and PLCH, but like in BHDS, they are usually located in the lower lung fields [12].
Other disease that can result in an image of lung cysts HRCT is amyloidosis. Amyloidosis is a heterogeneous group of disorders characterised by the deposition of specific fibrillar β-amyloid proteins in extracellular spaces. However, cystic changes in the lungs in amyloidosis are rare. Variously sized cysts with a well-formed wall often with calcifications are detected (Figure 11) [13].
Figure 11
Amyloidosis and lung involvement. Computed tomography scan, lung window, coronal plane. Bilateral pulmonary cysts of widely varying sizes, calcifications in cyst walls
Patients suspected of having BHDS, as in the presented cases, should undergo genetic testing to identify folliculin gene mutations, which is of pivotal importance because a positive test confirms clinical diagnosis and allows for genetic counselling.
Conclusions
We present the above-mentioned cases to draw attention to the possibility of BHDS occurrence in patients with apparent emphysematous cystic lesions unusually located in the lower and middle pulmonary fields, who also have positive history of pneumothorax, especially recurrent. Diagnosis of BHDS should be followed by pleurodesis when treating the first pneumothorax, and follow-up abdominal ultrasounds because of the risk of kidney malignancies.