Introduction
Nanoparticle-based drug delivery systems obtained significant consideration in the area of nanotechnology due to their unique characteristics, such as a large surface area, high reactivity, and improved solubility. These properties enable the efficient delivery of therapeutic agents to specific target sites, minimizing systemic toxicity and enhancing therapeutic efficacy. In particular, nanomaterials synthesized using bioactive components derived from plants have emerged as a natural and ecofriendly approach (Dillard and German, 2000). Various bioactive plant components, including curcumin (Hettiarachchi et al., 2021), quercetin (Parhi et al., 2020), kaempferol (Kazmi et al., 2021), rutin (Zhang and Han, 2018), berberine (Javed Iqbal et al., 2021), and others, have been employed for the synthesis of nanomaterials. However, curcumin nanoformulations have received the most extensive research and evaluation for various pharmaceutical applications.
One of the major reasons for extensive research on curcumin nanoformulations is the extremely low solubility and bioavailability of curcumin in its free form. Curcumin is a plant-based polyphenol compound derived from the rhizomes of Curcuma longa, commonly known as turmeric (Hewlings and Kalman, 2017). It possesses numerous pharmaceutical potentials, including anti-inflammatory (Peng et al., 2021), antioxidant (He et al., 2015), anticancer (Tomeh et al., 2019; Abd Wahab et al., 2020; Mirzaei et al., 2021; Danduga et al., 2022; Namwan et al., 2022), antimicrobial (Adamczak et al., 2020), and antimutagenic (Khan and Ahmad, 2020) effects. However, its therapeutic potential is hindered by its hydrophobic nature, which results in low systemic exposure and rapid clearance from the body.
To overcome these limitations associated with hydrophobic drugs, D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) is commonly employed for solubilization, emulsification, permeation, and stabilization in drug development (Zou and Gu, 2013; Gaonkar et al., 2017; Tan et al., 2017; Yang et al., 2018). TPGS has been recognized by the US Food and Drug Administration as a pharmacologically acceptable supplement for oral, parenteral, topical, nasal, and rectal/vaginal administration (Monteiro-Riviere et al., 2005; Van Hamme et al., 2006).
Earlier studies have demonstrated the safety of TPGS-based nanoparticles for oral administration and their extensive use in delivering curcumin to enhance its aqueous solubility (Vijayakumar et al., 2016; Meng et al., 2017). TPGS-Curcumin micelles, with a particle size of 12 nm, have been utilized to encapsulate curcumin, thereby improving its solubility and bioavailability, and enabling oral administration (Li et al., 2019). In this present study, we synthesized a curcumin nanoformulation (CN) with certain modifications, resulting in smaller formulations compared to the TPGS-Curcumin micelles synthesized by Li et al. (2019).
Chen et al. (2022) successfully synthesized curcumin-loaded nanoparticles using a blend of PLGA and TPGS, which exhibited significant anticancer activity against hepatocellular carcinoma cells. Another study conducted by Nguyen et al. (2021) demonstrated the antitumor activity of curcumin nanoparticles based on Polylactic acid-TPGS. In a study by Song et al. (2016), a solid dis-persion nanoformulation of curcumin-TPGS was synthesized to improve the aqueous solubility, dispersion ratio, bioavailability, and cellular absorption of curcumin.
This study aimed to synthesize a highly efficient CN with a smaller particle size, improved aqueous solubility, and enhanced physical stability compared to pure curcumin. The synthesized CN was subjected to comprehensive physicochemical characterization, and its in vitro anticancer activity was evaluated using colorectal cancer cells (HCT-116). The study highlights the potential of nanoformulations to enhance the dispersion of hydrophobic drugs like curcumin in water. Furthermore, it underscores the promising anticancer properties of curcumin, suggesting its potential application as a therapeutic agent in future anticancer treatments.
Materials and methods
Materials
Curcumin, TPGS, methanol, chloroform, and 0.1 μm filters were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany), while Nanopure water was provided by Ahmedabad University. Chemicals required for animal cell cultures, including Roswell Park Memorial Institute Medium (RPMI 1640), fetal bovine serum (FBS), trypsin-EDTA, phosphate buffer saline (PBS), dimethyl sulfoxide (DMSO), and 3-(4,5-Dimethylthiazol-2-yl)-2,5Diphenyltetrazolium Bromide (MTT), were procured from Himedia, India. The human colorectal cell line HCT-116 was obtained from NCCS, Pune, India. All materials utilized in the study were of pharmaceutical grade.
Synthesis of curcumin nanoformulation
The synthesis of CN was performed using the thin-film rehydration method, as described by Li et al. (2019), with slight modifications. In the initial stage of the synthesis, a 0.01% methanolic solution of curcumin was slowly added dropwise to a 0.1% TPGS solution (prepared in chloroform) at a stirring speed of 600–700 rpm and a temperature of 37°C using a magnetic stirrer (model: 10 MLH plus, REMI, Mumbai, India). The solvents were then evaporated, leaving behind a yellow film. Autoclaved Mili-Q water was used to resuspend the film and the resulting solution was centrifuged at 19 090 ×g for 25 min at 4°C using a refrigerated centrifuge (model: 5430R, Eppendorf, Hamburg, Germany). The upper translucent layer was separated, and to eliminate any free curcumin particles, the solution was filtered using a 0.1 μm filter. The final product obtained after filtration was considered the CN.
Lyophilization of synthesized CN
The synthesized CN exhibited stickiness, necessitating the use of a suitable cryoprotectant to reduce this stickiness during the lyophilization process. A 10% w/v mannitol solution was prepared, and one part of this solution was mixed with three parts of the synthesized product, using a 1 : 3 ratio. The mixture was then frozen at −80°C overnight and subsequently subjected to drying using a Vacuum Freeze Dryer (model: FD-10-MR, LABFREEZ, Beijing, China) at −40°C and 0.4 bar pressure for 24 h. The resulting lyophilized powder was stored at 25°C for further experimentation.
Solubility in water
Ten milligram of pure curcumin powder and synthesized CN were individually redispersed by dissolving them in 1 ml of nanopure water using separate transparent glass vials. This was achieved through gentle shaking and stirring. The visual observation method was employed to monitor the formation of aggregates or precipitates.
Particle size measurement
The particle size analysis of the synthesized CN was conducted using dynamic light scattering (DLS) with the Zetasizer Nano-ZS instrument (Malvern Panalytical, Malvern, United Kingdom). To perform the analysis, 1 mg/ml of synthesized CN was dissolved in nanopure water. Then, 10 μl of this suspension was diluted to a final volume of 1 ml for the analysis. The samples were prepared in triplicate for accurate measurements.
Zeta potential and polydispersity index
The zeta potential (ZP) and polydispersity index (PDI) of the CN solution were determined using the Zetasizer Nano ZS90 instrument (Malvern Panalytical, Malvern, United Kingdom) at a temperature of 25°C. To analyze the ZP, 1 ml of the CN solution was evaluated to determine the electrical charge of the solution.
Percentage of curcumin loading and encapsulation efficiency
To determine the curcumin loading and encapsulation efficiency (EE) of the synthesized CN, a range of curcumin concentrations (1–15 mg) were dissolved in methanol and added to 100 mg of TPGS solution, prepared in 5 ml of chloroform. The mixture was stirred at 600–700 rpm and maintained at 37°C until the solvent evaporated. The resulting dried film, containing the entire CN, was then solubilized in nanopure water and filtered using a 0.1 μm syringe filter to separate any non-encapsulated CN present. The filtrate was analyzed for further examination.
The optical density of the filtrate was measured using a spectrophotometer at 425 nm, allowing for the estimation of the quantity of free curcumin content in the filtrate. The curcumin loading and EE of the synthesized CN were quantified using the following equation (Zweers et al., 2003; Bisht et al., 2007):
Stability study
The synthesized CN was stored at a temperature of 4°C in a dark amber bottle for 8 months. At specific intervals of 5, 10, 15, 30, 60, 120, and 240 days, samples were withdrawn from the stored CN for particle size analysis. The stored CN sample was dissolved in nanopure water through manual shaking, without the need for additional sonication. The presence of curcumin particle precipitation indicates the instability of the synthesized CN, while a clear solution confirms its stability (Gou et al., 2011). The particle size of the synthesized CN was analyzed using the DLS method at various time intervals to assess any changes over time.
Field-emission scanning electron microscopy
The structure of the synthesized CN was examined using a field-emission scanning electron microscope (JSM7600F, Jeol, Tokyo, Japan). To prepare the sample, the lyophilized CN was mounted onto a copper-coated sample stub. The sample was then dried at room temperature and visualized under the FE-SEM at an acceleration voltage of 5.0 kV.
UV-visible spectroscopy
The optical property of pure curcumin and synthesized CN were analyzed by taking the absorption spectra between 200 and 800 nm and measuring maximum absorbance using a UV-visible spectrophotometer LMSP-UV1900 (Labman Scientific Instruments, Chennai, India).
Fourier transform infrared spectroscopy
Synthesized CN was characterized by the occurrence of a functional group using FTIR (Perkin-Elmer FTIR-1600, USA). Spectra were taken between 500 and 4000/cm.
X-Ray diffraction
The X-ray diffraction (XRD) spectrum of the synthesized CN was obtained using a SmartLab SE Multipurpose X-ray diffractometer (Rigaku, Tokyo, Japan) and analyzed using X’Pert high source software. This analysis aimed to analyze the crystalline structure of both curcumin and the synthesized CN.
To perform the XRD analysis, samples were placed on the sample holding area of a slide and inserted into the XRD instrument. The XRD patterns of the samples were collected at a temperature of 25°C using a Cu Kα X-ray radiation source. The scanning was conducted over a 2θ range from 10 to 70° with a step size of 0.02° and a scanning speed of 6.00°/min.
Furthermore, the average crystal size (d) of the CN was calculated using the Scherrer equation (Scherrer, 1918).
where λ is the wavelength of the X-ray, β is the full width at half maximum, and θ is Bragg’s angle of reflection.
In vitro release assay
For this assessment, 100 mg of lyophilized CN was dissolved in 10 ml of normal saline, and this solution was evenly distributed into 10 vials. The vials were then incubated at a temperature of 37°C for a maximum time of 48 h.
After the incubation period, the mixture was centrifuged at 19 090 × g for 3 min to separate the unencapsulated curcumin that remained in the nanoformulation. The free curcumin was obtained as a pellet and solubilized in methanol.
The absorbance of the solubilized curcumin was measured at a wavelength of 425 nm. The percentage release of curcumin was calculated using the provided formula.
In vitro anticancer activity of the synthesized CN
HCT-116 human colorectal cells were cultured in complete RPMI 1640 media supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml). The cell culture was maintained at a temperature of 37°C with 5% CO2 in a humidified environment.
Cytotoxicity analysis was conducted using the MTT assay, as described by Mosmann in 1983. Briefly, HCT-116 cells were seeded in a 96-well plate at a density of 104 cells/well and incubated overnight for cell attachment. After that, the cells were treated with various concentrations (ranging from 1.25 to 100 μM) of the synthesized CN, curcumin, TPGS, and the corresponding amount of cisplatin for a duration of 24 h.
Stock solutions of curcumin and cisplatin were prepared in DMSO at a concentration of 1% (v/v) and then diluted with RPMI 1640 media. Subsequently, 20 μl of MTT solution (5 mg/ml) was added to each well and incubated for 3 h. After incubation, 100 μl of DMSO was added to dissolve the formazan crystals formed. The optical density of each well was measured at 570 nm using a Microplate Spectrophotometer (Epoch BioTeck Instruments, Winooski, Vermont, United States). All experimental groups were analyzed in triplicate.
Statistical analysis
The obtained data were expressed as mean ± standard error based on triplicate experiments. To assess the significance of the results, the mean values of the treated groups were compared to the control group. Statistical analysis was performed using the ordinary one-way ANOVA test, utilizing GraphPad Prism 8.4.3 version. A P -value < 0.05 was considered statistically significant.
Results and discussion
Solubility test of CN
In this study, we successfully synthesized a water-soluble CN using the thin-film hydration method. The water solubility of pure curcumin was reported to be 0.0004 mg/ml (Yallapu et al., 2012), as evidenced by the presence of insoluble microflakes on the surface of nanopure water and at the bottom of the glass vial (Fig. 1A). However, the synthesized CN was finely dispersed in water (Fig. 1B) due to encapsulation in TPGS and the formation of a nanoformulation. The solubility of curcumin was significantly increased up to 10 mg/ml in the synthesized CN compared to pure curcumin, corresponding to a 2.5 × 104-fold enhancement in solubility. It was observed that the water solubility of the synthesized CN was ~ 13 times higher than TPGS-stabilized curcumin nanoparticles (Rachmawati et al., 2016). This indicated that the water solubility of the synthesized CN was superior to TPGS-stabilized curcumin nanoparticles. Consequently, it can be inferred that curcumin becomes highly dispersible and soluble in an aqueous medium after encapsulation in the nanoformulation, leading to an increase in bioavailability (Bosselmann and Williams, 2012). Thus, the synthesized CN effectively addressed the major limitation of curcumin, which is poor water solubility. Additionally, the increased water solubility of curcumin after nanoformulation reduces the requirement for solvents and lowers the risk of solvent toxicity without the need for large amounts of solvents.
Particle size, PDI, and ZP
To confirm the synthesis of CN and analyze its particle size, PDI values, and ZP, the dynamic light scattering method was employed. The results revealed that the average particle size of the synthesized CN was 6.2 ± ± 1.9 nm with a PDI value of 0.164 (Fig. 2A). The ZP of the synthesized CN was −10.1 ± 3.21 mV (Fig. 2B). The particle size of the synthesized CN was approximately 50% smaller than previously synthesized TPGS-curcumin nanoparticles (12.3 ± 0.1 nm) (Montes-Burgos et al., 2009). The PDI value of the synthesized CN fell within the range of 0.0 to 1.0, indicating a homogeneous population of the nanoformulation (Chen et al., 2011; Badran, 2014; Putri et al., 2017). Additionally, the ZP value of the synthesized CN was comparable to the ZP values of −12.50 and −13 mV reported in studies by Rachmawati et al. (2013) and Luiz et al. (2022), respectively. This similarity can be attributed to the presence of ionizing groups in the structure of TPGS. Previous studies have shown that curcumin-TPGS mixed micelles, synthesized using an improved film dispersion technique, had a particle size of 65.54 ± 2.57 nm, a PDI value of 0.114 ± 0.027, and an average ZP value of −14.90 ± 2.50 mV, demonstrating increased stability in dispersion (Ji et al., 2018). It has been reported that a ZP value of approximately −15 mV exhibits greater accumulation at the tumor site and extended retention time in the blood compared to nanoparticles with more negative or positive charges (Faraji and Wipf, 2009). However, it should be noted that the stabilization of particles by electrostatic repulsion is typically effective only when the ZP is above ± 30 mV, raising questions about the stabilization of the synthesized CN solely based on the ZP (Mengual et al., 1999; Gao et al., 2010). Previous studies have highlighted that the size of nanoparticles affects tissue distribution and membrane permeability of the drug of interest (Islam et al., 2017). Due to their large surface area, high drug loading capacity, controlled drug release, biocompatibility, and smaller particle size, nanoformulations have demonstrated a significant impact on the management and prognosis of various diseases for in vivo applications (Soo Choi et al., 2007). Therefore, the findings suggest that the smaller particle size, significant PDI value, and ZP of the synthesized CN make it suitable for further in vivo applications.
Drug loading percentage and encapsulation efficiency percentage
The percentage of curcumin loading at different curcumin amounts (2–14 mg) in 100 mg of TPGS is presented in Fig. 3A. The study demonstrated that the synthesized CN achieved a loading capacity of 10% for curcumin (Fig. 3A) with an EE of 80% (Fig. 3B), which was higher than other curcumin-loaded TPGS nanoformulations reported in the literature. For example, a curcumin-loaded TPGS nanoformulation achieved 8.17% loading of curcumin and 85% EE (He et al., 2010). Curcumin loaded in Pluronic P123 micelles exhibited only 4% loading capacity and 46% EE (Ganguly et al., 2017), while curcumin loaded in casein micelles showed 4% loading capacity and 81% EE (Pan et al., 2014). Another study reported 3.24% loading and 83% encapsulation of crizotinib using TPGS (Wang et al., 2022). TPGS has been shown to enhance EE and the amount of released curcumin at the targeted site during targeted cancer therapy (Sun et al., 2021). High drug-loading capacity is advantageous as it reduces the amount of nanoformulation required to achieve therapeutic efficacy, minimizing drug overdose and production costs associated with anticancer drug delivery nanoformulations (Shen et al., 2017). Therefore, the synthesized CN shows promise as a potential agent for the delivery of curcumin in anticancer therapy.
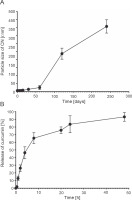
Stability study
The stability of the synthesized CN was evaluated by measuring the particle size after storage at room temperature for up to 2 months. Figure 4A shows that the synthesized CN particles maintained a uniform size without any aggregation during the 2-month storage period. In comparison, curcumin-encapsulated nanoparticles synthesized by Dai et al. (2019) exhibited a particle size of approximately 103 nm and storage stability for only 1 month at room temperature. After 2 months of storage, we observed a gradual increase in the particle size of the synthesized CN. According to Stoke’s law, the particle diameter is proportional to the sedimentation rate (McClements, 2015). Consequently, smaller particles exhibit slower movement and are less prone to accumulation. Based on this understanding, we hypothesize that the smaller particle size of the synthesized CN contributes to its long-term stability of up to 2 months without aggregation.
Field-emission scanning electron microscopy
The FE-SEM analysis of the synthesized CN revealed needle-shaped surface crystal structures, as depicted in Fig. 5A. This surface morphology is consistent with previous studies on curcumin nanocrystals obtained through the precipitation method (He et al., 2010; Rajasekar and Devasena, 2015; Ndong Ntoutoume et al., 2016). According to Junghanns and Müller (2008), nanocrystals are colloidal systems characterized by highly stable nanosized cores surrounded by surfactant molecules. Nanocrystals offer several advantages, including improved stability, enhanced drug loading capacity, costeffectiveness, and improved dissolution rates for hydrophobic drugs (Wang et al., 2013; Danhier et al., 2014). The needle-shaped structure observed in the synthesized CN can be attributed to its increased water solubility (Sharifi et al., 2020). These advantages have contributed to the growing popularity of nanocrystals as an innovative delivery system for poorly water-soluble phytochemicals such as curcumin.
UV-Visible spectroscopy
The UV-Visible spectral analysis demonstrated that the synthesized CN exhibited a similar absorption peak at a wavelength of 425 nm, comparable to pure curcumin (Fig. 5B). This finding is consistent with the absorption spectra of curcumin-loaded nanoparticles synthesized by Wang et al. (2018a), which also exhibited a prominent absorption peak at 425 nm. This similarity in absorption maxima indicates that curcumin was successfully entrapped during the synthesis of the nanoformulation. It suggests that the chemical composition of curcumin remained intact throughout the synthesis process, with no significant structural changes occurring in the curcumin molecule. Moreover, this observation implies that curcumin retains its biochemical properties and pharmacological potential even after being incorporated into the CN formulation.
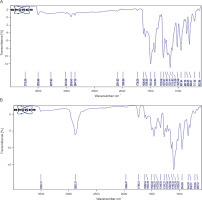
Fourier transform infrared spectroscopy
The FTIR spectra (Fig. 6B) of the synthesized CN exhibited characteristic peaks corresponding to the functional groups present in curcumin, which were also observed in the FTIR spectrum of pure curcumin (Fig. 6A). These characteristic peaks include vibrations at 1025.49 and 1029.02/cm (C−O −C stretching), 1273.37 and 1276.27/cm (aromatic C−O stretching), 1424.64 and 1423.82/cm (olefinic C−H bending), 1626.45 and 1626.54/cm (C=C vibrations), 2847.32 and 2882.31/cm (C−H methyl ring), and 3503.89 and 3502.74/cm (phenolic O−H stretching). These findings are consistent with previous studies by Rompicharla et al. (2017), Wang et al. (2018b), and Hettiarachchi et al. (2021) which also reported the presence of these functional groups in curcumin. The presence of these characteristic functional groups in the synthesized CN confirms that there were no significant structural alterations in the curcumin molecule during the synthesis process. Therefore, the synthesized CN can be considered a suitable drug carrier or nanoformulation for in vitro and in vivo experiments, as it protects the curcumin molecule from disintegration and preserves its biochemical properties.
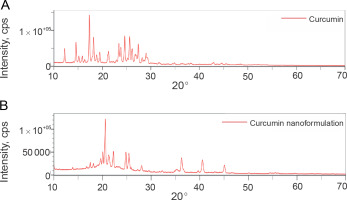
X-ray diffraction
The XRD analysis was conducted to examine the crystalline structure of pure curcumin and the synthesized CN. In both cases, the XRD patterns exhibited high peak intensities in the range of 10–30 positions at a diffraction angle of 2θ, indicating the crystalline nature of curcumin (Fig. 7A) and curcumin present in the synthesized CN (Fig. 7B). The similarity in peak intensities between curcumin and curcumin in the synthesized CN suggests that the curcumin molecule was preserved and not degraded during the production of the nanoformulation (Peng et al., 2018; Taghavifar et al., 2022).
Additionally, the average crystallite domain size of the synthesized CN was calculated to be 7.87 nm using the relevant formula. This value is in close proximity to the particle size measured by DLS analysis, indicating that the synthesis protocol resulted in the production of significantly smaller nanoformulations without altering the structural characteristics of curcumin.
In vitro release profile of the synthesized CN
The in vitro release profile of curcumin from the synthesized CN was evaluated at 37−C using a saline solution with a pH of 7.4. The release profile, as shown in Fig. 4B, demonstrated that the synthesized CN exhibited an initial burst release of approximately 25% of curcumin within the first 4 h. This initial burst release may be attributed to the curcumin molecules that were adsorbed on the surface of the nanoformulation.
Following the initial burst release, the release profile demonstrated a sustained and stable release of curcumin for up to 10 h. After 24 h, the cumulative release of curcumin reached approximately 80%. This release profile indicates that the synthesized CN has a higher curcumin release compared to the study that reported only 50% curcumin release after 24 h (Rachmawati et al., 2016).
Anticancer activity of the synthesized CN
The anticancer activity of the synthesized CN was evaluated using the MTT assay, and the results, as shown in Fig. 8A, demonstrated that the synthesized CN significantly inhibited the growth of HCT-116 cells compared to the control (untreated) and pure curcumin. The IC50 value of the synthesized CN after 24 h of treatment was found to be 12.74 ± 0.54 and 32.33 ± 3.52 μM, respectively (Table 1). While the IC50 value of TPGS and cisplatin (positive control) for 24 h were 41.984 ± 2.62 and 13.32 ± 0.35 μM, respectively.
Table 1
The IC50 values of treated groups on the HCT-116 cell line at 24 h
Fig. 8
The % cell viability was determined by MTT assay after the treatment of various concentrations (1.25–100 μM), curcumin (Group I), TPGS (Group II), CN (Group III), and cisplatin (Group IV) on HCT-116 cells at 24 h (A); IC50 values were plotted with mean ± SE; *P < 0.001, **P < 0.0001 when Groups II, III, and IV were compared with Group I; NS – non significant when Group III was compared with Group IV (B)

Comparing the IC50 values, it was observed that the synthesized CN exhibited a significantly lower IC50 value (P < 0.001) compared to TPGS, whereas it was significantly higher (P < 0.0001) as compared to synthesized CN and cisplatin. The IC50 value of synthesized CN (12.74 ± 0.54 μM) was almost similar to the metal-based anticancer drug cisplatin (13.32 ± 0.35 μM) which indicated that the synthesized CN more significantly inhibited the cell growth with similar anticancer activity of cisplatin (Fig. 8B). This suggests that the combination of CN and cisplatin with their similar effects may increase the efficacy of therapeutic response and decrease the mortality cancer patients (Sandhiutami et al., 2021). The IC50 value of CN optimized by Wadhwa et al. (2014) was noted as 20.32 μM on the HT-29 colorectal cancer cell line which is a higher IC50 value than the synthesized CN on HCT-116. One of the primary goals of this study was to put a step forward in developing an effective CN as an appropriate strategy for either primary or supplementary cancer treatment.
Overall, the proven anticancer potential of the synthesized CN supports its further exploration for biomedical and pharmaceutical applications.
Conclusion
In summary, the successful synthesis of TPGS-based crystalline CN using nanotechnology has provided several advantages, including smaller particle sizes, good storage stability, and rapid dispersibility in aqueous media. These characteristics enable the effective delivery of curcumin, a hydrophobic molecule, and enhance its anticancer activity.
The curcumin loading and EE results indicate that the synthesized CN is an efficient delivery system for curcumin. This study proved that synthesized CN enhanced the anticancer activity of curcumin in vitro. Moreover, the results demonstrated the potential of synthesized CN as a therapeutic approach for the management of various cancers, and supplementary research is necessary to determine its clinical potential.