Introduction
Dendritic cell (DC) therapy was once considered one of the strategies for treating patients with advanced cancer. Dendritic cells, known as the most professional antigen-presenting cells (APCs), build a link between adaptive and innate immune responses. DCs can capture, process, and present peptide antigens, including exogenous and endo- genous antigens in the context of MHC class I and class II molecules, to CD8+ and CD4+ T cells, respectively [1, 2]. As a heterogeneous population, human DCs include resting-state DCs, inflammatory DCs, and Langerhans cells [3]. Resting-state DCs consist of conventional DC1 (cDC1), con- ventional DC2 (cDC2), and plasmacytoid DCs. Human conventional DCs type 1 (cDC1), also called CD141+ DC or BDCA3+ DC, have superior ability to stimulate allogeneic or autologous naive CD4+ T cells and in vivo cross-presentation by stimulating the proliferation of CD8+ T cells as well as induction of cytotoxic T cells [4-6]. In general, CD141+ DC cDC1 plays a vital role in antigen uptake from liver tumor cells, trafficking the antigens into the lymph node and cross-presenting antigens to CD8+ T cells, which promotes an anticancer immune response [7-10].
Because natural DCs and cDC1 account for only about 1% and 0.3% of all human peripheral blood mononuclear cells (PBMCs), respectively, DC differentiation and maturation remain challenging [11]. The in vitro development of DCs depends on the culture methods and the combination of the stimuli; hence, various methodologies to culture functional DCs from human peripheral blood have been published. In the early 1990s, human hematopoietic stem cells (HSCs) and umbilical cord blood (UCB) became attractive sources of DC precursors for potential clinical use in the culture condition of GM-CSF [8, 12, 13]. Still, the HSCs remained a rare and specialized source of DCs, and the cell culture cycle was too long. The seminal discovery that immune-stimulating myeloid DCs can be generated from PBMCs by culturing granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 4 (IL-4), followed by maturation stimuli, was first reported in the 1990s, which is called the classical culture method, providing the basis for numerous dendritic cell clinical trials, especially in cancer immunotherapy [14, 15]. Following maturation in media containing tumor necrosis factor α (TNF-α), CD40 ligand, lipopolysaccharide (LPS), or type-I interferon (IFN-α and IFN-β), conventional immature DCs were used [16, 17]. In general, DC identification including several essential phenotypes includes major histocompatibility class IIhigh (MHC II), major histocompatibility class I (MHC I), and lineage marker CD11c (in some subsets in human DCs), co-stimulatory molecules CD80 and CD86, and the maturation status marker CD83.
Numerous substitutions have been reported later to reduce the cycle for preparing mature DCs in vitro, collectively known as the fast DC models, which are highly comparable to DCs derived from the original GM-CSF and IL-4 culture methods. It is much less labor-intensive and more efficient to manufacture. One of the usual methods involves CD14+ cells isolated from PBMC treated with GM-CSF and IL-4 for one day, followed by maturation stimuli (TNF-α, IL-1α, IL-6, or PGE2) for another 24 h. The maturation stimuli generate highly uniform fast-DC, whereas DC-induced T cell proliferation is enhanced over time [18]. Another study found that combining IFN-g with LPS allowed for a higher level of IL-12p70 to be detected. MoDCs treated with clinical-grade cytokines and IFN-g also improve polarization efficiency in serum-free conditions [19]. However, DCs cultured by these methods ultimately proved ineffective for cancer immunotherapy because of the defects in the cytotoxic T cells they induced [20, 21]. There is also a fast-DC model of the human monocyte culture method with GM-CSF and IL-15 for 2 days followed by a classic combination of proinflammatory cytokines compared with TLR7/8 agonist-based maturation stimuli for 24 hours [22, 23]. Although these strategies have been published, clinical trials are still lacking.
We developed a fast model with IL-4 and IFN-β for 24 hours, followed by the addition of non-specific antigenic stimuli, such as keyhole limpet hemocyanin (KLH), LPS, or inactivated human immunodeficiency virus (HIV)-1, which induced the monocytes to differentiate into mature DCs after 3 days (4B-DCs) [24]. However, 4B-DCs could induce stronger activation and proliferation of allogeneic CD8+ T cells than conventional GM-CSF and IL-4 induced DCs (hereafter referred to as G4-DCs), with most of the 4B-DCs going into apoptosis from day 4. In addition, the maturation regents, such as LPS and KLH, could not be used for human beings. To prepare functional DCs for clinical use, we optimized the protocol of DC generation by adding GM-CSF to improve the viability (Supplementary Figure 1) and using GMP grade poly I:C and IL-1β for DC maturation (hereafter referred to as G4B-DCs). In this study, we found that compared with the G4-DCs, G4B-DCs expressed higher levels of CD86 and HLA-DR, produced a higher level of TNF-α, and showed a higher ability to promote allogeneic naive CD4+ T cell and CD8+ T cell proliferation and IFN-γ production. Furthermore, G4B-DCs induced the proliferation of Flu-M1-specific CD8+ T cells.
Material and methods
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. All experiments using human samples were approved by the Ethics Committees of Guangxi University of Chinese Medicine (approval number: GXUCM IRB H 2017-12-02-1). Informed consent was obtained from every participant.
Reagents
RPMI Medium 1640 (Catalog# 11875; Solarbio, Beijing, China) was supplemented with 10% fetal bovine serum (FBS; Catalog# 10099141; Gibco, Waltham, MA). 1 × PBS (Catalog# P1020) was purchased from Solarbio (Beijing, China). Bovine Serum Albumin (Catalog# 9048-46-8) was purchased from Sigma-Aldrich (Darmstadt, Germany). EDTA, disodium salt, dihydrate (Catalog# A100105) was purchased from Sangon Biotech (Shanghai, China). Recombinant human IL-4 (Catalog# 200-04), GM-CSF (Catalog# AF-300-03), IFN-β (Catalog# 300-02BC), IL-1β (Catalog# 200-01B), and IL-2 (Catalog# 200-02) were obtained from PEPROTECH (Cranbury, NJ). Poly I:C (Catalog# tlrl-pic) and lipopolysaccharide (LPS-EB; Catalog# tlrl-eblps) were obtained from InvivoGen (San Diego, CA). Human CD14 positive selection kitII (Catalog# 17858), human CD8 positive selection kitII (Catalog# 17853), human naïve CD4+ T cell negative isolation kits (Catalog# 17555), human T cell isolation kit (Catalog# 17951), and Lymphoprep (Catalog# 07851) were obtained from STEMCELL Technologies (Vancouver, Canada). FITC conjugated mouse anti-human CD86 (Clone FUN-1), APC conjugated mouse anti-human CD83 (Clone HB15e), APCconjugated mouse anti-human CD8 (Clone RPA-T8), FITC gated mouse anti-human CD8 (Clone HIT8a), FITC conjugated rat anti-human CCR7 (Clone 3D12), PE conjugated mouse anti-human CD11c (Clone 3.9), APC conjugated anti-human HLA-DR (Clone G46-6), FITC conjugated anti-human HLA-A2 (Clone RPA-T8), and PE conjugated mouse anti-human CD3 (Clone UCHT1) were obtained from BD Pharmingen (San Diego, CA). DAPI Staining Solution (Catalog# C1005) was obtained from Beyotime Biotechnology (Shanghai, China). Human TruStain FcX (Catalog# 564219) was obtained from BioLegend (San Diego, CA). Human IL-12 p70 High Sensitivity ELISA Kit (Catalog# abs510012), IL-10 High Sensitivity ELISA Kit (Catalog# abs551808), TNF-α ELISA Kit (Catalog# abs510006), and human IFN-γ High Sensitivity ELISA Kit (Catalog# abs551817) were purchased from Absin (Shanghai, China). The Vybrant carboxyfluorescein succinimidyl ester (CFSE) cell proliferation tracking kit (Catalog# HY-D0938) was bought from MedChemExpress (Shanghai, China). HLA-A*02:01 NY-ESO-1 SLLMWITQC-APC (Catalog# TB-M011-2) and HLA-A*02:01 Influenza M1 Tetramer-GILGFVFTL-APC (Catalog# TS-0012-2C) were purchased from MBL (Tokyo, Japan).
Preparation of 3-day cultured DCs and conventional DCs
Peripheral blood mononuclear cells were isolated from peripheral blood from healthy adult volunteer donors by density gradient centrifugation on Lymphoprep centrifuged at 1500 rpm for 20 min. Monocytes were recovered as much as possible at the interface and washed three times in PBS containing 2% autologous plasma and 2 mmol/ml EDTA. The CD14+ monocytes were purified from the suspension using the Human CD14 Positive selection kit and resuspended with a concentration of 1 × 106 cells/ml in RPMI medium with 10% FBS. The cell suspension was divided into 0.5 ml each and cultured in the 48-well plate (BD Pharmingen) in the presence of GM-CSF (50 ng/ml), IL-4 (10 ng/ml), and IFN-β (5 × 103 U/ml) at 37oC in a 5% CO2 humidified incubator for 48 hours. Then the IL-1β (10 ng/ml) and poly I:C (20 µg/ml) were added as maturation stimuli, culturing for 24 hours to promote maturation. The G4-DCs were cultured in the presence of GM-CSF (50 ng/ml) and IL-4 (10 ng/ml) in a 5% CO2 humidified incubator for 5 days. Then the IL-1β (10 ng/ml) and poly I:C (20 µg/ml) were added, culturing for 48 hours to promote maturation. The G4B-DCs and G4-DCs were harvested at the same time.
Fluorescence imaging
Dendritic cells in an immature and mature state were harvested and washed in PBS containing 0.1% BSA twice at room temperature. We typically used a 100 µl experimental sample for each sample, and the samples were incubated in 5 µl of Human TruStain FcX for 15 min at 4oC according to the instructions. The cell suspension was then cultured with FITC conjugated mouse anti-human CD86, PE conjugated mouse anti-human CD11c, and APC conjugated anti-human HLA-DR for 30 min at 4oC avoiding light. Then after washing the cells with PBS containing 0.1% BSA twice, cells were resuspended in 4% paraformaldehyde (PFA) and mixed with DAPI Staining Solution in a 1 : 1 ratio. Fluorescence imaging was performed using Axio Observer 7, and the images were analyzed using ZEN 3.2 (blue edition).
Phenotype of G4B-DCs and G4-DCs
Aliquots of the G4B-DCs to be analyzed were harvested and washed in PBS containing 0.1% BSA twice at room temperature. We typically used 1 × 106 cells in a 100 µl experimental sample, and the samples were incubated in 5 µl of Human TruStain FcX for 15 min at 4oC according to the instructions. The cell suspension was then cultured with fluorescent-labeled antibody (mouse anti-human HLA-DR, mouse anti-human CD83, mouse anti-human CD86, mouse anti-human HLA-A2, anti-human rat CCR7, shown in Fig. 1) for 30 min at 4oC protected from light. Then after washing the cells with PBS containing 0.1% BSA twice, cells were resuspended in 4% PFA before flow cytometry analysis. Based on the G4B-DC regular phenotype assay results, DCs were divided into three groups: control, LPS (10 µg/ml), poly I:C, and IL-1β (20 µg/ml and 10 ng/ml). Cells were treated as described for G4B for 5 days and stimulated by different stimuli for 48 h.
Fig. 1
Morphology and phenotype of G4B-DCs. CD14+ cells purified from human PBMCs by a positive selection method (8 × 105 cells/ml) were cultured in a 48-well plate, and two types of DCs were harvested simultaneously. DCs were stimulated by poly I:C and IL-1β or LPS to detect the DC-related phenotype differentiation. A) The morphology of G4B-DCs and G4-DCs was observed under a phase-contrast upright microscope at an original magnification of 10×. B) Fluorescent images of imG4B-DCs (top panel) and mG4B-DCs (bottom panel) which were incubated with DAPI Staining Solution, FITC conjugated mouse anti-human CD86, PE conjugated mouse anti-human CD11c, and APC conjugated anti-human HLA-DR. Magnification = 63×, scale bars = 5 mm C) Flow cytometry facilitated the phenotypic characterization of G4B-DCs and G4-DCs. Two types of DCs were examined for cell surface expression of CD11c, CD86, HLA-A2, HLA-DR, CD83, as described in the Material and methods section. Percent-positive cells and MFI are presented

Stimulation of allogeneic naïve CD4+ T cells and CD8+ T cells
The naïve CD4+ T cells and CD8+ T cells were isolated from normal human PBMCs using the human naïve CD4+ T cell isolation kit and human CD8-positive isolation kit, respectively. Naïve CD4+ T cells and CD8+ T cells were co-cultured with allogeneic G4B-DCs and G4-DCs separately in the RPMI medium with exogenous IL-2 in 96-well U-bottom plates in a volume of 20 U/ml. Cultures were performed in triplicate. According to the manufacturer’s protocol, cell proliferation was assessed by using CFSE-labeling naïve CD4+ T cells and CD8+ T cells. The naïve CD4+ T cell and CD8+ T cell proliferation was assessed by flow cytometry analysis.
Cytokine production analysis
The supernatant was collected before cells were stained with fluorescent-labeled antibodies and stored at –20oC until the IL-12p70, IL-10, TNF-α, and IFN-γ release assay. A commercially available Enzyme-Linked Immunosorbent Assay kit purchased from Absin was used according to the manufacturer’s protocol.
Induction of antigen-specific CD8+ T cells
Flu-M1 (58-66, GILGFVFTL) was loaded on G4B-DCs for 2 h before co-culturing with allogeneic T cells. Unlabeled T cells were isolated by a T cell isolation kit and transmitted into the 96-well U bottom plate. 2 × 105 cells were used in a 100 µl experimental sample per well. G4B-DCs were washed in RPMI 1640 medium containing 10% FBS twice. DCs were added to T cells at a ratio of 1 : 5 in the RPMI 1640 medium supplemented with IL-2 (20 U/ml) in the 96-well U-bottom plate for 6 days. The proliferation of antigen-specific CD8+ T cells was assessed by flow cytometry labeling mouse anti-human CD8 (BD Pharmingen) and iTAg MHC tetramer (MBL).
T cells were resuspended at a concentration of 2 × 107 cells/ml per tube. 5 µl of human TruStain FcX was added to each test tube and incubated for 15 min at 4oC. Then 10 µl of HLA-A*02:01 Influenza M1 Tetramer-GILGFVFTL-APC was added per tube and vortexed gently. Samples were incubated for 60 min at 4oC protected from the light followed by adding 20 µl of FITC mouse anti- human CD8 per tube and incubated at 4oC for 30 min, protected from the light. Another tetramer, HLA-A*02:01 NY-ESO-1 SLLMWITQC-APC, was added together with FITC mouse anti-human CD8 to the test samples, which were incubated for 30 min at room temperature protected from light. All these samples were resuspended with 3 ml of PBS containing 0.1% BSA after the incubation and centrifuged at 400 g for 5 min. Aspirating the supernatant, cells were resuspended in 0.5% PFA for at least 1 h (no more than 24 h) before the flow cytometry analysis. The equipment of flow cytometry analysis was BD C6 plus, and the data were processed with Flowjo10.6.2.
Results
Morphology and phenotype differences between 7-day G4-DCs and G4B-DCs
The morphological differences observed between the G4-DCs and G4B-DCs are presented in Figure 1A. G4B-DCs showed the most optimal morphology on day 3 of culture; they were smaller than G4-DCs and appeared to have aggregative morphology. The fluorescent imaging of imG4B-DCs and mG4B-DCs was next examined separately. Both mature and immature G4B-DC expressed the lineage marker CD11C, antigen-presenting molecule HLA-DR and co-stimulatory molecule CD86 (Fig. 1B). However, under our experimental conditions, the expression of the DC mature molecule CD83 was not detected on both kinds of DCs (data not shown).
In order to analyze the exact phenotype differences based on the morphology and confocal fluorescence imaging difference, flow cytometry was used for detection. Both types of DCs highly express CD11c, HLA-DR, and CD86. The MFI of HLA-DR and CD86, as well as the percentage of CD83-positive cells in G4B-DCs from donor A, are significantly higher compared with the G4-DCs, while the expression of HLA-A2 on G4B-DC is significantly lower compared to their G4-DC counterparts (Fig. 1C). Similar to donor A, the MFI of HLA-DR in donor B is significantly higher than their G4-DC counterparts, the expression of CD86 is weaker than conventional DCs, and the percentage of CD83-positive cells is similar in both types of DCs (Fig. 1D).
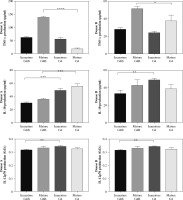
Cytokines secreted by G4B-DCs and G4-DCs
We next measured the expression levels of TNF-α, IL-10, and IL-12p70 to analyze the functions of G4B-DCs and G4-DCs in immature and mature states, respectively. As shown in Figure 2, in donor A, only mature G4B-DCs cells secreted higher levels of TNF-α compared to conventional DCs, immature G4B-DCs, and G4-DCs secreted comparable amounts. Mature G4-DCs secreted much less of the TNF. In contrast, in donor B, mature G4B-DCs secreted high levels of TNF-α, higher than mature G4-DCs. Both G4B-DCs and G4-DCs in mature form secreted higher levels of TNF-α than immature forms. Immature G4B-DC and G4-DC secreted similar levels of the tested cytokine. The levels of IL-10 secreted by G4B-DCs were lower than those secreted by G4-DCs only in donor A; in donor B, IL-10 levels in mature G4B-DCs were lower than those in immature G4-DCs but slightly higher or equal compared with mature G4-DCs. The level of IL-12p70 secretion by G4B-DCs is similar to that of G4-DCs. However, a statistically significant difference was found between immature forms, where lower concentrations were observed in immature G4B-DCs.
Fig. 2
Cytokines secretion by G4B-DCs. Supernatants of the two types of DC cultures were examined for the levels of TNF-α, IL-10, and IL-12 p70 by ELISA. The cytokines produced by immature or mature DCs were compared in parallel. For statistical analysis, Dunnett’s t-test was used; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001
Naïve CD4+ T cell activation and proliferation by G4B-DC and G4-DC
The standard mixed lymphocyte reaction (MLR) examined the biological effects of G4B-DCs on naïve CD4+ T cells. Purified naïve CD4+ T cells were labeled with CFSE and co-cultured with either G4B-DCs or G4-DCs at a T to DC ratio of 2 : 1, 10 : 1, and 50 : 1 for 7 days. Levels of cell proliferation by CFSE-labeled T cells were assessed by flow cytometry. The proliferation percentage of naïve CD4+ T cells from G4B-DCs is two to ten times higher than that of G4-DCs at different ratios. It is worth noting that G4B-DCs showed an outstanding ability of naïve CD4+ T cell proliferation at a T to DC ratio of 2 : 1 in both donors, which indicates the remarkable potential of G4B-DCs in activating T cells’ immune responses (Fig. 3A, B).
Fig. 3
Proliferation and activation of naïve CD4+ T cells stimulated by G4B-DCs or G4-DCs. CFSE-labeled purified naïve CD4+ T cells were co-cultured with allogeneic IL-1β and poly I:C matured G4B-DCs or G4-DCs at a DC to T ratio of 1 : 2, 1 : 10, 1 : 50 in RPMI medium containing IL-2 (20 U/ml) in a 96-well U-bottom plate for 6 days. Proliferation of the CFSE-labeled donor A (A) and donor B (B) naïve CD4+ T cells was assayed by flow cytometry. The frequency of proliferating cells of naïve CD4+ T cells is shown C) IFN-γ production by DC-activated naïve CD4+ T cells. For statistical analysis, Dunnett’s t-test was used; *p < 0.05, ***p < 0.001
To further determine the activation status of these naïve CD4+ T cells stimulated by allogeneic G4-DCs or G4B-DCs, the secretion level of IFN-γ in the supernatants of these cultures on day 7 was quantitated by ELISA. In Figure 3C, the IFN-γ level of G4B-DC from donor A was four times higher than that of its G4-DC counterpart (p = 0.0001, two-tailed Student t test). In conclusion, T cells and G4B-DCs in the 1 : 2 ratio were significantly more effective at inducing IFN-γ than other G4B-DC and G4-DC ratios.
CD8+ T cell stimulation by G4B-DC and G4-DC
The ability of G4B-DC to induce activation and proliferation of allogeneic CD8+ T cells was then examined. Purified (> 90%) CD8+ T cells labeled with CFSE were co-cultured with G4B-DC or G4-DC at T to DC ratios of 2 : 1, 10 : 1, and 50 : 1 for 6 days. The assessment of CD8+ T cell proliferation stimulated by DCs was detected by flow cytometry on day 7. At a ratio of 1 : 2, the G4B-DC-induced CD8+ T cell proliferation was two times more effective than that induced by G4-DCs (Fig. 4A). The capacity of the G4B-DC at a ratio of 1 : 10 or 1 : 50 was two to three times, or even six times, higher than the G4-DCs (Fig. 4A, B).
Fig. 4
Proliferation and activation of CD8+ T cells stimulated by G4B-DCs and G4-DCs. CFSE-labeled purified CD8+ T cells were co-cultured with allogeneic IL-1β and poly I:C matured G4B-DCs or G4-DCs at a DC to T ratio of 1 : 2, 1 : 10, 1 : 50 in RPMI medium containing IL-2 (20 U/ml) in a 96-well U-bottom plate for 6 days. Proliferation of the CFSE-labeled donor A (A) and donor B (B) CD8+ T cells was assayed by flow cytometry Proliferation and activation of CD8+ T cells stimulated by G4B-DCs and G4-DCs. CFSE-labeled purified CD8+ T cells were co-cultured with allogeneic IL-1β and poly I:C matured G4B-DCs or G4-DCs at a DC to T ratio of 1 : 2, 1 : 10, 1 : 50 in RPMI medium containing IL-2 (20 U/ml) in a 96-well U-bottom plate for 6 days. Proliferation of the CFSE-labeled donor A (A) and donor B (B) CD8+ T cells was assayed by flow cytometry. The frequency of proliferating cells is shown C) ELISA quantitated IFN-γ production by non-labeled CD8+ T cells in the culture supernatants. The data shown are representative of one of three independent experiments with a standard deviation of < 10%. Each experiment utilized two different blood donors and the data depict the mean ± SD derived from three samples. ND means not detected

ELISA was used to examine the levels of IFN-γ in the culture supernatant. Figure 4C shows that CD8+ T cells activated with allo-G4B-DCs produced significantly more IFN-γ than those stimulated with G4-DCs. At a 1 : 2 ratio, the IFN-γ secretion level of G4B-DC was more than four times that of G4-DC. These findings may speak eloquently of G4B-DC’s remarkable ability to stimulate CD8+ T cells.
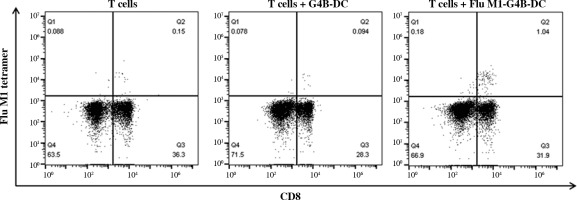
G4B-DC induced Flu-M1 (58-66, GILGFVFTL)-specific CD8+ T cells in vitro
We investigated whether G4B-DCs were effective in inducing antigen-specific CD8+ T cells. Flu-M1 (58-66, GILGFVFTL) was chosen as a model antigen. G4B-DC was loaded with antigens two hours before the maturation stimuli and incubated at 37°C. Autologous T cells were cocultured with matured antigen-loaded DCs for 7 days. The proliferation of antigen-specific CD8+ T cells was assessed using APC labeled MHC tetramer and anti-human CD8 at day 7. The antigen-loaded G4B-DC induced 1.04% of Flu-M1 (58-66)-specific CD8+ T cells, ten times more than the non-antigen-loaded control (Fig. 5).
Fig. 5
Antigen-specific CD8+ T cell induction by G4B-DC. G4B-DCs were loaded with Flu-M1 (58-66, GILGFVFTL) for 2 hours before the maturation stimuli, autologous CD8+ T cells were co-cultured with antigen-loaded mature G4B-DCs at a ratio of 5 : 1 in a 96-well U bottom plate for 6 days in the presence of IL-2 (20 U/ml), antigen-specific CD8+ T cells were stained with APC-labeled flu M1 tetramer and detected by flow cytometry on day 7
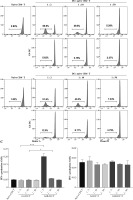
Up-regulation of CD141 and XCR1 expression on G4B-DCs stimulated by poly I:C and IL-1b
G4B-DC has a strong ability to stimulate CD8 T cells, so we speculated that it might belong to the cDC1 subtype. We investigated the expression level of the cDC1 marker CD141, CD370, and XCR1 on G4B-DC and G4-DCs. As is shown in Figure 6A, B, compared with immature G4B-DCs, the expression of CD141 and XCR1 was up-regulated on poly I:C with IL-1β matured G4B-DCs. In contrast, on G4-DCs, CD141 expression was down-regulated by LPS or poly I:C with IL-1β stimulation. These data suggest that some G4B-DCs may belong to the cDC1 subtype, but further confirmation is needed.
Fig. 6
Up-regulation of CD141 and XCR1 expression on G4B-DCs stimulated by poly I:C and IL-1b. G4B-DC and G4-DC from donor A and donor B were induced as described in Figure 1; expression levels of CD141/XCR1 (A) and CD141/CD370 (B) were detected by flow cytometry; percentage of positive cells is shown Up-regulation of CD141 and XCR1 expression on G4B-DCs stimulated by poly I:C and IL-1β. G4B-DC and G4-DC from donor A and donor B were induced as described in Figure 1; expression levels of CD141/XCR1 (A) and CD141/CD370 (B) were detected by flow cytometry; percentage of positive cells is shown

Discussion
Dendritic cell therapy was once considered one of the effective strategies for treating cancer. However, most of the clinical trials did not meet the expectations of the scientists. One suggested reason was the limited capacity of migration and antigen presentation of PBMC-produced moDCs in ex vivo. Compared to the conventional protocols for the generation of mature G4-DC in vitro, we now evaluated a novel strategy for differentiating monocyte-derived DCs in 72 h of in vitro culture, including 48 h cultured in the presence of GM-CSF, IL-4 as well as IFN-β followed by maturation stimuli of poly I:C and IL-1β cultured for another 24 h. G4B-DC was discovered to have a superior ability to activate T cells and induce antigen-specific CD8+ T cells in vitro, suggesting that it could be a promising vaccine strategy for cancer immunity.
The significant difference between G4B-DCs observed under the microscope at 10× is that G4B-DCs were smaller in size. They formed large clumps just loosely attached to the bottom in culture compared with the G4-DCs (Fig. 1A), which may be due to the different adhesion molecules expressed by the two types of DC. It is worth mentioning that the MFI of CD86 from G4B-DC is about 25% higher than that of G4-DC, and the MFI of HLA-DR from G4B-DC is nearly four times higher than that of G4-DC (Fig. 1C, D), implying that G4B-DC has better antigen presentation and stimulation activities. However, both DCs only expressed a tiny quantity of CCR7, which could influence their homing capacity in vivo and necessitates more research.
The cytokine production patterns (Fig. 2) convincingly showed that G4B-DCs produced a higher relative level of TNF-α. IL-10 cytokine expression in G4B-DC is lower than in G4-DC. Of note, poly I:C and IL-1β maturation stimuli successfully increased the cytokine level of immature G4B-DC after stimulation. Our findings show that combining poly I:C and IL-1β as maturation stimuli results in reasonable TNF-α and IL-10 secretion, which may play a physiological role in regulating antigen presentation by DCs.
A striking finding is the outstanding ability of G4B-DC to induce the proliferation of naïve CD4+ T cells and CD8+ T cells. As shown in Figure 3A, B, the G4B-DCs induced 4-6-fold higher levels of naïve CD4+ T cells at a ratio of 1 : 2, which is possible because the HLA-DR expression of G4B-DCs was 2-4-fold higher than that of G4-DCs. G4B-DCs cultured in a 1 : 2 ratio with CD8+ T lymphocytes induced 2-fold higher levels of lymphocytes than did their G4-DC counterparts, as clearly shown in Figure 4A, B. The proliferation of CD8+ T cells is probably caused by the G4B-DCs’ higher levels of expression of CD86 and CD83. Both naïve CD4+ T cell and CD8+ T cell proliferation showed a dose-effect relationship at the ratio of 1 : 2, 1 : 10, and 1 : 50, respectively. In line with previous findings, we found the same pattern of IFN-γ secretion in G4B-DC or G4-DC co-cultured with nal¨ve T cells. These data demonstrate that G4B-DC has the potential ability to promote the proliferation of T cells, which probably present not only MHC class I molecules but also MHC II molecules [25].
Another characteristic of mature DCs is their ability to stimulate specific T-cell responses. To assess the capacity of G4B-DCs to induce antigen-specific T cell responses, G4B-DCs were loaded with Flu-M1 (58-66, GILGFVFTL). Antigen-loaded DCs were co-cultured with autologous T cells in the presence of IL-2 (20 U/ml). T cells and T cells co-cultured with unloaded G4B-DCs were used as controls. Flu-M1-specific CD8+ T cells account for 1.04% after one IL-2 stimulation after 6 days, which may be applicable in clinical DC-based immunotherapy trials for such virally infected patients.
The previous study showed only 30% of GM-CSF and IL-4-induced DCs expressed CD141 on day 8 [26]. CD141 was represented in over 90% of immature G4-DCs. Surprisingly, it decreased in response to stimulation with LPS, poly I:C, and IL-1β. The expression levels of CD141 and XCR1 in G4B-DCs stimulated by poly I:C and IL-1β were twice as high as those in immature DCs, and some supporting G4B-DCs might belong to the cDC1 subtype, which explains the strong cross-expression ability of G4B-DCs to some extent. But further proof is needed.
DC-based vaccines often use a 5-7 day DC protocol of generation in general, known as the standard DC. To shorten the incubation period and generate a more effective functional DC, we developed this 3-day DC generation strategy. Researchers have reported the fast strategy of DCs generated within 3-4 days, which outputs DCs with low expression of IL-12p70 and the ability to induce antigen-specific CD8+ T cells [27-29]. Another study found that CCR7 expression was low after 24 hours of culture with GM-CSF and IL-4, followed by another 24 hours with maturation stimuli, which could affect immune surveillance and tissue homeostasis [30, 31]. CD83, an immunoglobulin protein that serves as a DC maturation marker, promotes the expression of MHC II and CD86 molecules on the cell surface, thereby inhibiting IL-10-driven DC degradation [32, 33]. The DCs generated in our system showed a similar trend compared with the above research. The main shortage in our system is probably due to the lack of CCR7, which may cause feedback on DC migration and poor responsiveness in some patients.
Here, we report that human CD14+ cells can be differentiated into functional, mature DCs in 3 days. G4B-DCs highly express MHC class I, II, and CD86, and secrete a high level of TNF-α. In terms of allogeneic CD4+ T and CD8+ T cell proliferation and IFN-γ production, G4B-DCs outperformed conventional G4-DCs. More importantly, G4B-DCs have the remarkable ability to induce Flu-M1-specific CD8+ T cells. G4B-DCs may be a potential candidate for clinical immunotherapy.


