Introduction
Immunoglobulin A nephropathy (IgAN) is a form of chronic glomerulonephritis which is currently considered the most common primary glomerulopathy worldwide, leading to renal failure in 20–50% of adult patients at 20 years. In the paediatric population, end-stage renal disease (ESRD) was shown to develop in 11% of patients at 15 years [1]. Depending on the severity of clinical symptoms and histopathological findings, glucocorticosteroids and immunosuppressive drugs may be used in the treatment of IgAN. Similarly to any chronic disease, IgAN may affect patient functioning and wellbeing due to the limitations it imposes. IgA nephropathy in the paediatric population affects mostly school-age children and adolescents, which means that a chronic disease becomes an additional burden during the key developmental period which is already psychologically challenging by itself. Health-related quality of life (HRQoL), a parameter describing human wellbeing in relation to health-related factors (physical, mental, and social health), is currently considered an important factor indicating a therapeutic success in chronic disease and a predictor of patient morbidity and mortality. Tools to evaluate HRQoL in adult patients are well established, while appropriate psychometric tools in the paediatric population have only been developed during the last decade. In IgAN, both the disease course with periods of remission and exacerbation and the need for hospitalization, and the treatment used, leading to isolation from peers and a change in body physique, may be factors that have a significant negative effect on the patient’s quality of life. Available literature lacks data on HRQoL in children with IgAN.
In our study, we attempted to evaluate: (1) HRQoL in children and adolescents with IgAN compared to the reference Polish population of healthy children, (2) the effect of social factors on HRQoL in the study group, and (3) personal competence and severity and expression of anger as factors affecting patient’s self-perception and health-related behaviours, which may be important in the context of future medical care following transition to adulthood.
Material and methods
This multicentre cross-sectional study included 51 of 140 patients in the Polish IgAN Registry (2000–2015) in whom the disease developed in childhood. The patients were under the care of 7 paediatric nephrology centres in Poland.
Inclusion criteria were: patient age ≥ 8 years, no disease exacerbation during the last 3 months, and written informed consent for study participation.
We excluded mentally retarded children and those with the duration of kidney disease < 6 months. The study was approved by the bioethics committee at the Medical University of Warsaw.
Psychometric analysis was performed using the following tools:
Kidscreen-52 questionnaire – a Polish version of the universal European questionnaire to evaluate HRQoL in children and adolescents. This tool has been developed for children aged 8–18 years, healthy or suffering from disease. It measures ten dimensions of HRQoL: (1) physical well-being, mental health as evaluated in three dimensions: (2) psychological well-being, (3) moods and emotions, and (4) self-perception, and social functioning as evaluated in five dimensions: (5) autonomy, (6) parent relations and home life, (7) social support and peers, (8) school environment, (9) social acceptance (bullying), and (10) financial resources. Questions are answered using standardized 5-grade evaluation regarding frequency (never, seldom, quite frequently, frequently, always) or intensity (not at all, slightly, moderately, much, very much). The results were shown as the overall score from 0 to 100. This questionnaire was validated in the population of Polish children and adolescents (healthy and with chronic illnesses) and reference ranges were developed for age- and gender-stratified groups. The instrument can be used as a generic instrument to assess quality of life in children and adolescents with a chronic illness [2].
Anger Expression Scale (Skala Ekspresji Gniewu, SEG) [3] – a tool to evaluate the severity of anger and its expression in common situations. It is a universal tool which can be used for both healthy and ill children. This tool has been developed for children aged 11–17 years. It includes 20 items scored from 1 (never) to 5 (always), grouped in two subscales of 10 items each related to expressed and suppressed anger. The overall score is the sum of scores for individual items, calculated separately for expressed and suppressed anger. The results are compared to the mean normalized values stratified for age, gender, and social background. Raw results are transformed to standardized units (stens), where 1–4 stens indicate low intensity of anger, and 7–10 stens indicate high intensity of anger.
Personal Competence Scale (Skala Kompetencji Osobistej, KompOs) [4] developed by Zygfryd Juczyński – a scale measuring general perception of personal competence grouped into subscales A and B. Subscale A measures perceived strength necessary to initiate actions, and subscale B measures persistence in continuing actions. Each subscale includes 6 items with 4 possible answers. Each item is scored from 1 to 4, each subscale from 6 to 24, and the overall score ranges from 12 to 48, with higher values indicating higher perceived personal competence, strength and persistence. Upon transformation to standardized units (stens), 1–4 stens (overall score 12–30) are rated low, and 7–10 stens (overall score 37–48) are rated high. This tool has been developed for group and individual evaluation of children and adolescents in conditions of health and disease. Internal consistency (Cronbach’s α) is 0.74, and reliability is 0.51. Reference values for the Polish paediatric population aged > 11 years were developed for this questionnaire. Our results were compared with the reference values for healthy peers.
In our study, we evaluated results obtained using the Kidscreen-52 questionnaire and the SEG and KompOs scales. We also developed a questionnaire that included items related to the previous disease course, treatment used, patient education, and family situation to evaluate the effect of these factors on HRQoL in children with IgAN.
Statistical analysis
Normal distribution of the variables was evaluated using the Shapiro-Wilk and Lilliefors tests. Normally distributed data were shown as mean values ± standard deviation (SD), and non-normally distributed data were shown as medians and ranges. Statistical analysis included the Student t test and linear regression. P < 0.05 was considered statistically significant. Analyses were performed using the Statistica 9.0 software (StatSoft).
Results
The study group included 32 boys and 19 girls at the mean age of 14.54 ±3.69 years. The mean duration since the diagnosis of IgAN was 4.98 ±3.9 years (0.8–10.3), significantly longer in girls (6.58 ±4.2 vs. 4.03 ±3.40 years, p < 0.05). Clinical characteristics of the study group are shown in Table I. Among children in the study group 4 had coexisting diseases – epilepsy in 1 patient, allergy was diagnosed in 2 patients, arrythmia in 1, congenital heart defect in 1. Anthropometric data of the study group revealed that mean BMI was 21.87 kg/m2 (13.87–30.42), obesity was diagnosed in 8 patients and underweight in 2 children.
Table I
Clinical characteristics of the study group
Clinical manifestations of IgAN included haematuria with coexisting proteinuria in 39 (76.49%) patients. Among them 26 children had macroscopic and 13 microscopic haematuria. Isolated microscopic haematuria was noted in 4 (7.8%) and isolated proteinuria in 6 (11.76%) children. In 3 patients (5.8%) IgA nephropathy manifested as nephritic syndrome with kidney injury. Hypertension was present in 12 (23.53%) children. Glucocorticosteroid therapy was used in 36 (70.59%) children, including previous steroid therapy (3-month interval before the study time) in 36 (70.59%) children and current steroid therapy in 15 (29.41%) children. 10 (19.61%) children were on immunosuppressive therapy during the study time. Immunosuppressive medications used were: mycophenolate mofetil in 2 children, azathioprine in 8 and cyclosporine A in 2 patients. Mean eGFR in the study group was 107.25 ml/min/1.73 m2 (48.8–178.97). Decreased eGFR (< 90 ml/min/1.73 m2) was found in 6 children (11.76%).
Of 51 children in the study group, 29 (56.86%) resided in an urban area and 22 (43.14%) resided in a rural area. Due to renal disease and its treatment, 7 of 51 children required an individualized education programme. Immediate household care was most commonly provided by the mother (37 children), and less frequently by both the mother and father (8 children), the father (one child), or grandparents (one child; no response to this question was provided in the remaining cases). Paid employment was reported by 66% of caretakers, and 34% were not working.
The results of the Kidscreen-52 questionnaire, evaluating HRQoL, are shown in Table II. We found that physical wellbeing in children with IgAN was rated worse (p < 0.005) in comparison to healthy peers. Both boys and girls with IgAN also rated their psychological wellbeing as significantly worse compared to reference values in boys and girls (p < 0.05). The dimensions of self-perception and social support and peers were rated significantly worse by boys in the study group compared to the population reference values. Social acceptance was rated higher in children with IgAN than in healthy peers; the difference was statistically significant among girls.
Table II
Kidscreen-52 questionnaire findings in the study group
| Parameter | Study group (n = 51) | Reference values | Boys (n = 32) | Reference values in boys | Girls (n = 19) | Reference values in girls | P in comparison to reference values |
|---|---|---|---|---|---|---|---|
| Mean score ± SD | |||||||
| Physical well-being | 62.84 ±20.25 | 70.21 ±17.351 | 65.78 ±21.44 | 72.71 ±18.503 | 57.89 ±17.51 | 66.54 ±19.52 | 10.055; 3< 0.05 |
| Psychological well-being | 66.83 ±18.86 | 68.02 ±18.78 | 69.53 ±18.41 | 78.05 ±17.294 | 62.28 ±19.22 | 75.30 ±19.087 | 4,7< 0.05 |
| Overall mood | 77.31 ±17.07 | 71.55 ±16.67 | 77.68 ±18.39 | 81.00±18.70 | 76.69 ±15.03 | 76.24 ±18.19 | |
| Self-perception | 69.71 ±21.71 | 69.67 ±20.65 | 71.09 ±20.90 | 78.85 ±17.785 | 67.37 ±23.41 | 68.39 ±22.22 | 5< 0.05 |
| Leisure time – autonomy | 67.84 ±19.86 | 66.05 ±19.80 | 70.16 ±19.94 | 75.41 ±19.77 | 63.95 ±19.62 | 70.36 ±21.71 | |
| Relations with parents | 78.27 ±19.14 | 73.27 ±19.71 | 77.86 ±20.01 | 80.02 ±18.08 | 78.95 ±18.08 | 77.91 ±20.28 | |
| Financial resources | 69.44 ±28.41 | 56.11 ±27.682 | 65.10 ±28.51 | 71.83 ±26.64 | 76.75 ±27.44 | 70.92 ±26.72 | 2< 0.05 |
| Social support and peers | 62.91 ±23.17 | 64.62 ±21.40 | 63.54 ±21.56 | 73.03 ±19.376 | 61.84 ±26.25 | 74.38 ±19.45 | 6< 0.05 |
| School environment | 62.99 ±20.88 | 56.78 ±20.02 | 61.20 ±20.78 | 65.45 ±21.33 | 66.01 ±21.26 | 67.24 ±20.47 | |
| Social acceptance | 91.67 ±16.67 | 86.34 ±18.83 | 88.80 ±19.70 | 88.53 ±16.69 | 96.49 ±8.017 | 89.24 ±16.36 | 7< 0.05 |
Financial resources in the study group were rated as significantly higher compared to the population reference values.
Among manifestations of IgAN, a significant effect of proteinuria on HRQoL was found. The presence of proteinuria was associated with significantly worse physical wellbeing as evaluated using the Kidscreen-52 questionnaire compared to the absence of proteinuria (58.72 ±18.45 vs. 74.44 ±22.97; p < 0.05). The presence of erythrocyturia and hypertension had no effect on HRQoL. Analysis of the treatment effect on HRQoL showed that current steroid therapy and treatment with azathioprine or cyclophosphamide (within the last 3 months) had no effect on various dimensions of HRQoL in the study group. No correlation was found with HRQoL and patients’ age or duration of the disease.
No significant differences were found when the results of the Kidscreen-52 questionnaire were compared between urban and rural area residents (Table III).
Table III
Kidscreen-52 questionnaire findings in relation to the place of residence
We found no effect of the education mode (regular schooling versus individualized education programme) on HRQoL.
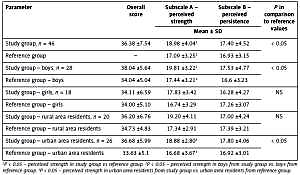
Evaluation using the KompOs scale (Table IV) was carried out in 46 children from the study group > 11 years of age and showed that perceived personal competence was rated high by 23 (50%) children in the study group, moderate by 14 (30.44%) children, and low by 9 (19.56%) children. Compared to the population reference values, children with IgAN were characterized by significantly higher perceived strength, which was shown in the overall study group and among boys. Perceived strength was also higher among urban area residents in the study group compared to the reference values for urban area residents. Perceived persistence in the study group did not differ compared to the population reference values. We found a positive correlation between the KompOs subscale A score (strength to initiate actions) and relations with parents (r = 0.32, p < 0.05) and school environment (r = 0.29, p < 0.05) dimensions of the Kidscreen-52 questionnaire.
Table IV
Personal competence (KompOs) scale findings
| Parameter | Overall score | Subscale A – perceived strength | Subscale B – perceived persistence | P in comparison to reference values |
|---|---|---|---|---|
| Mean ± SD | ||||
| Study group, n = 46 | 36.38 ±7.54 | 18.98 ±4.041 | 17.40 ±4.52 | < 0.05 |
| Reference group | – | 17.09 ±3.251 | 16.93 ±3.15 | |
| Study group – boys, n = 28 | 38.04 ±5.64 | 19.81 ±3.222 | 17.53 ±4.77 | < 0.05 |
| Reference group – boys | 34.04 ±5.04 | 17.44 ±3.212 | 16.6 ±3.23 | |
| Study group – girls, n = 18 | 34.11 ±6.59 | 17.83 ±3.42 | 16.28 ±4.27 | NS |
| Reference group – girls | 34.00 ±5.10 | 16.74 ±3.29 | 17.26 ±3.07 | |
| Study group – rural area residents, n = 20 | 36.20 ±6.76 | 19.20 ±4.11 | 17.00 ±4.24 | NS |
| Reference group – rural area residents | 34.73 ±4.83 | 17.34 ±2.91 | 17.39 ±3.21 | |
| Study group – urban area residents, n = 26 | 36.68 ±5.99 | 18.88 ±2.803 | 17.80 ±4.06 | < 0.05 |
| Reference group – urban area residents | 33.63 ±5.1 | 16.68 ±3.673 | 16.92 ±3.01 | |
Using the SEG scale (in 46 children older than 11 years), we observed high intensity of expressed anger in 9 (19.56%) children in the study group, moderate intensity in 9 (19.56%) children, and low intensity in 28 (60.88%) children (Table V). High intensity of suppressed anger was observed in 27 (58.70%) children in the study group, moderate intensity in 13 (28.26%) children, and low intensity in 6 (13.04%) children. Compared to the reference values, children with IgAN were characterized by a significantly lower intensity of expressed anger (p < 0.001) and a significantly higher intensity of suppressed anger (p < 0.01).
Table V
Anger Expression Scale (SEG) findings
| Parameter | Expressed anger | Suppressed anger | P in comparison to reference values |
|---|---|---|---|
| Mean ± SD | |||
| Study group, n = 46 | 24.11 ±7.491 | 32.19 ±6.642 | < 0.0011; < 0.0012 |
| Reference group | 27.65 ±6.811 | 28.06 ±7.072 | |
| Study group – boys, n = 28 | 23.04 ±7.21 | 32.57 ±6.64 | NS |
| Reference group – boys | 27.61 ±6.82 | 28.35 ±7.07 | |
| Study group – girls, n = 18 | 25.78 ±7.84 | 31.61 ±6.79 | NS |
| Reference group – girls | 27.68 ±6.08 | 27.31 ±7.09 | |
| Study group – rural area residents, n = 20 | 24.47 ±7.873 | 32.95 ±6.404 | < 0.053; < 0.014 |
| Reference group – rural area residents | 27.27 ±6.483 | 27.14 ±6.414 | |
| Study group – urban area residents, n = 26 | 24.38 ±6.965 | 32.42 ±5.756 | < 0.055; < 0.016 |
| Reference group – urban area residents | 27.8 ±6.485 | 28.40 ±7.286 | |
3 Expressed anger in study group (rural area residents) vs. reference group from rural area p < 0.05.
4 Suppressed anger in study group (rural area residents) vs. reference group from rural area p < 0.01.
These relations were found for both urban and rural area residents. We also found that the severity of expressed anger correlated negatively with relations with parents (r = –0.38, p < 0.05) and school environment (r = –0.34, p < 0.05) dimensions of the Kidscreen-52 questionnaire. The severity of expressed anger also showed negative correlations with both subscales of the KompOs scale, i.e. perceived strength (subscale A: r = –0.32; p < 0.05), and perceived persistence (subscale B: r = –0.38; p < 0.05).
Discussion
Previous studies on quality of life in patients with renal disease focused on patients with ESRD, treated with dialysis therapy, or following kidney transplantation [5–8]. We are aware of two studies that evaluated HRQoL in children with less severe (stage 2–4) chronic kidney disease (CKD) [5, 9], including one in the Polish population [5]. A few studies concerned the HRQOL in children with nephrotic syndrome [10, 11] or chronic glomerulonephritis [12]. Our study is the first to evaluate HRQoL in a homogeneous paediatric population with IgAN. We studied patients from 7 paediatric nephrology centres in Poland, and thus this group may be considered representative for the Polish population of children with IgAN.
We used the Kidscreen-52 questionnaire to evaluate HRQoL as it is a universal tool that conforms to high methodological standards. It is the only tool with reference values available for the Polish population, as it has been validated in a representative population of 1718 Polish children aged 8–18 years [2].
We found significantly worse psychological wellbeing in our study group compared to healthy peers. This is an important finding in the context of normal renal function in nearly all studied children, as it indicates that quality of life in children with kidney disease is reduced prior to the diagnosis of renal failure. Significantly worse HRQoL in regard to physical and mental functioning compared to healthy peers has already been observed in children with ESRD [6]. In this population, it tended to be worse among patients treated with dialysis therapy compared to those after kidney transplantation or managed medically [5, 6]. Also in studies that evaluated HRQoL in children with stage 2–4 CKD, quality of life in children with the disease was significantly worse compared to healthy children. In addition, Gerson et al. reported that HRQoL was not related to glomerular filtration rate, and no differences in HRQoL were found between stages 2–4 of CKD [9]. Reduced psychological wellbeing in our patients may be caused by the consciousness of chronic disease, which may lead to the necessity of dialysis in the future, and significantly influence the lifestyle. The other important factor causing reduced psychological wellbeing is isolation from colleagues as a result of hospitalizations.
Physical wellbeing was rated significantly worse in boys with IgAN in comparison to healthy peers. A similar tendency was noted among girls, but probably because of the small number of girls enrolled in the study, the difference was not statistically significant. Worse physical wellbeing in patients with IgAN can be caused by symptoms of the disease such as oedema, episodes of recurrent haematuria, as well as adverse effects of pharmacotherapy such as overweight, weakness, and susceptibility to infections.
Gerson et al. found that in children, older age and longer disease duration correlated with better overall HRQoL. This is probably connected with education concerning dealing with chronic disease. In our study duration of the disease and patients’ age had no effect on HRQoL [9].
In our study, more negative scores in specific dimensions of HRQoL in comparison to reference ranges were noted in boys. Boys were also characterized by worse perceived physical wellbeing, self-perception, and relations with peers. This may be related to reduced participation in peer activities, sport activities and competitions, and increased school absence. Similarly, Quist et al. in a study that evaluated quality of life in children after kidney transplantation found worse somatic complaints and increased social problems (peers, school, hobbies) in boys compared to healthy peers [13]. In our study group, quality of life might have been reduced due to the treatment used and associated adverse effects such as body weight increase and short stature, related mostly to steroid therapy. In a study that evaluated quality of life in children with steroid-sensitive nephrotic syndrome, which was primarily treated with steroids, reduced quality of life in regard to social functioning was found [14]. Similarly, a negative effect on quality of life was found in children with other chronic diseases who required glucocorticosteroid therapy, such as asthma and haematological disorders [15, 16]. In our study group with IgAN, analysis of the treatment effect on HRQoL showed that current steroid therapy had no effect on various dimensions of HRQoL. In children with IgAN glucocorticosteroid therapy doses are lower than used in hematologic disorders, adverse effects may be less significant and the impact on QoL may be smaller.
In addition, awareness of a chronic disease itself is a major psychological burden regardless of the disease severity.
The lack of statistical significance of differences between results scored by girls with IgAN in comparison to the reference ranges may be caused by the small number of girls enrolled in the study.
We found no effect of the mode of education (individualized vs. regular schooling) on the evaluated HRQoL dimensions. This might have been related to the small size of the study sample.
No effect of the area of residence (rural vs. urban) was found on HRQoL. This is a positive fact, which may be connected with similar conditions of living, advances in communicate technology and sufficient access to health care, which causes that burden caused by the disease does not cause differences in quality of life between children from different areas. Similarly, Kiliś-Pstrusińska, who evaluated a population of Polish children with stage 2–3 CKD, found no differences in quality of life in relation to social environment, gender, or age [5].
Literature data indicate that perceived personal competence is a major factor affecting quality of life, self-perception, and health-related behaviours. High perceived personal competence and control over unpleasant life events reduce emotional excitement and increase readiness to parry threats, thus helping to cope [17]. In our study group, as many as half of the patients declared high perceived personal competence, and 27.45% rated it as moderate. In the study by Silva et al. [18], a high level of perceived personal competence correlated positively with compliance with chronic immunosuppressive therapy following kidney transplantation. A similar relation was reported by Alatawi et al. in a group of patients with diabetes type 2 who required chronic drug therapy [19].
Both overexpressed anger and a strong tendency to suppress anger have an adverse health effect. Anger expression, which begins to develop already in the pre-school age, is an established factor affecting cardiovascular disease risk, and chronically suppressed anger may underlie psychosomatic disorders [20]. In our study group, we found a clear trend to suppress anger compared to the healthy population. The importance of such behaviour is not clear in patients with IgAN, but we might expect its influence on patients’ compliance as a tendency to suppress emotions favours communication problems. Similar behaviours in patients with chronic disease were reported by Phipps et al. [21], who concluded that anger suppression is typical for children with chronic disease. However, anger suppression predisposes to non-adherence behaviours, and thus it is important to identify it in clinical practice using such tools as the SEG scale.
In conclusion, we found lower HRQoL in regard to physical and psychological wellbeing in a group of Polish children with IgAN compared to healthy peers. These findings suggest that HRQoL should be monitored in this patient group, and clinicians should address these issues as an indicator of effective care. Measures should also be taken to improve HRQoL in this patient group, including provision of psychological care.