Introduction
Breast cancer is the second most common cancer in women (after skin cancer). It is also considered as the most prevalent cause of mortality in women worldwide [1]. Breast cancer treatment is very costly, comprising various methods of chemotherapy, radiotherapy, or brachytherapy [2]. Screening is the first strategy for reducing breast cancer mortality and treatment cost [3].
Mammography is the standard screening method that has reduced breast cancer mortality in the last decades. However, the number of cancers that cannot be detected with mammography is substantial, especially in dense-breasted women [4,5]. Mammography sensitivity in evaluating breast mass in the dense breast is as low as 30-48%. Studies also show that the chance of breast cancer between screenings was almost 18 times higher in very dense breasts compared with fatty breasts [6].
In the last year the American Cancer Society (ACS) sug-gested magnetic resonance imaging (MRI) as a screening tool for very high-risk cases. However, MRI is expensive and might cause risks due to the need for contrast media. Moreover, MRI has a lower specificity for breast cancer screening in comparison to mammography, so it has a higher rate of false positives, and consequently it needs further MRI follow-up [7].
There is a direct correlation between increased breast density and higher interval cancer rates, and there is a worse prognosis for these clinically detected cancers. Subsequently, it can deduce that dense breast tissue itself is a risk factor for breast cancer. Thus, techniques as a complement to mammography are required, principally for women with dense breasts [8].
Breast sonography can be a useful and powerful screening tool because it is widely accessible, easy to perform, free of detrimental side effects, and it does not require radiation or contrast agents. Studies have also revealed that performing breast sonography for patients with breast pain can decrease pain and anxiety [9].
Also, studies have shown that application of supplementary screening tools such as breast sonography increases the chance of detection of early node-negative invasive breast cancer in women with dense breast tissue.
The incidence rates of breast cancer in Asia are lower than in Europe and the United States; however, breast density is noticeably higher in Asian than in African American and white women. It implies that a different diagnostic routine of screening breast sonography should be considered in Asian women [10].
The purpose of this study is to evaluate the application of whole-breast sonography in the assessment of breast lesions in women with dense breast tissue, and to estimate its accuracy in comparison with mammography. We also investigated different factors that might influence the prevalence of different breast lesions.
Material and methods
During December 2015 and March 2016, 207 asymptomatic women participated in this study. The breast tissue densities of participants were designated as categories 3 or 4, using the Breast Imaging Reporting and Data System (BI-RADS). High-resolution ultrasonography of the breast was performed for all patients after mammography and physical examination.
All breast sonographies were performed by an experienced radiologist. The same radiologist who reviewed the screening mammography also performed screening sono-graphy examinations.
All the mammograms were reviewed before screening sonography. The breast density was determined as stated by the gradation of the American College of Radiology (ACR) BI-RADS protocol on a scale from A to D. If the findings on mammography were normal and the breast density was defined as grades C-D, the patients were considered as appropriate cases for screening sonography.
The procedure was explained to all the patients in detail by the examining breast radiologist and when the patients received the results of the screening mammography. The patients provided verbal consent.
Prior to sonography, a physical examination was performed with appropriate anatomical positioning for all patients by the radiologist. The existence of a palpable abnormality led to the exclusion of the patient from the study.
All sonographies were performed with ATL 3000 or 5000 units by using 5-12 MHz bandwidth electronically focused transducers. Both breasts were thoroughly exa-mined with overlapping radial and anti-radial, vertical and horizontal scans. To ensure the complete scan of all breast tissue, the retro-areolar region was scanned with angled views. depending on the size and texture of the breasts, the procedure time was varied between 4 and 15 min (mean: 7 min). The unit was not equipped with elastography.
The sonographic findings were categorised as BIRADS I (no focal lesions), BIRADS II (fibrocystic change and simple cyst), BIRADS III (probably benign including complicated cyst, ductal ectasia, fibroadenoma), and BIRADS IV and V (suspicious or probably malignant lesions). In cases with more than one lesion, the final assessment was categorised as the highest BIRADS.
A questionnaire was used to gather information about the risk factors related to the prevalence of breast cancer. The risk factors included the duration of oral contraceptive pill (OCP) usage, the frequency of pregnancy, frequency of breastfeeding, previous history of cancer, and history of breast cancer in first- and second-degree relatives.
The χ2 or t-test was performed to analyse the results statistically. Appropriate Pearson correlation was carried out to determine the relationships between the variables. The p-value of less than 0.05 considered as statistically significant.
Results
A total of 207 women aged 32-62 years, mean ± SD 43.7 ± 6.5 years, participated in this study. Out of a total 207 performed mammographies, 152 cases (73.4%) had dense and 55 cases (26.6%) had very dense breast. None of the cases had a positive history of malignancy, while 19% of them had a positive record of breast cancer in first- or second-degree relatives. Table 1 shows the prevalence of the sonography findings. According to Table 1, 115 cases were normal, and there were 100 findings in 92 cases.
Table 1
The prevalence of different sonography findings
| Sonography result | Normal | Benign lesion* | Complicated cyst | Ductal ectasia | Fibroadenoma | Cancer |
|---|---|---|---|---|---|---|
| Number | 115.0 | 81.0 | 4.0 | 6.0 | 8.0 | 1.0 |
| Percentage | 55.5 | 39.1 | 2.9 | 1.9 | 3.9 | 0.5 |
The benign lesions included fibrocystic change, simple cyst, and cluster microcyst (Figures 1 and 2); the prevalence of these lesions separately were 38, 37, and 6, respectively. The majority of our subjects had normal and benign breast lesions. All cases with ductal ectasia had bilateral lesions (Figure 3). Nine cases underwent short-term follow-up at 6, 12, 24, and 36 months without any biopsy and just by mammography and sonography.
Figure 1
A) An asymptomatic woman with mammographically dense breast. B) Simple cyst in ultrasound image of the same patient
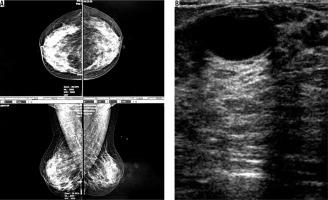
Figure 2
A) An asymptomatic woman with mammographically dense parenchymal tissue. B) Fibrocystic changes in ultrasound image of the same patient
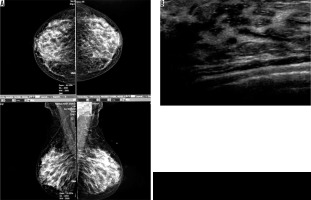
Figure 3
A) An asymptomatic mammographically dense breast. B) Ductal ectasia in ultrasound image of the same patient
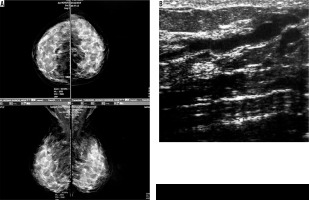
Four cases had complicated cyst with no changes in size and no other new lesion during the follow-up time (Figure 4). Five cases were typically fibroadenoma (i.e. well-circumscribed, homogenous hypo echo, round to ovoid, without calcification or posterior shadowing and any changes during follow-up time) (Figure 5). Four cases underwent core needle biopsy sampling, of which three had “unshaped margin” or “indistinct border”, the signs of malignancy in sonography, and one had a large size despite its typical morphological sign of fibroadenoma. Biopsy result showed fibroadenoma in three of them and cancer in one case (Figure 6).
Figure 4
A) An asymptomatic woman and monographically dense breast. B) Stable cyst containing fine echoes (complex cyst) during follow-up ultrasounds of the same patient
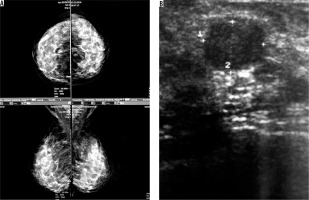
Figure 5
A) An asymptomatic woman with mammographically dense breast (ACR C). B) Ultrasound reveals a stable, well-circumscribed, homogenously hypo echoic, ovoid lesion typical for fibroadenoma
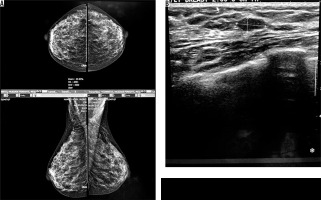
Figure 6
An asymptomatic patient referred for screening mammography. A) Mammographically dense parenchyma with no distinct mass or parenchymal distortion. B) Hypoechoic mass with irregular borders at 6 o’clock in the right breast with 3 cm distance to nipple. Invasive ductal carcinoma was reported in tissue diagnosis of the same patient
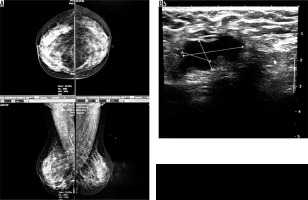
Table 2 shows the prevalence of different factors related to breast cancer.
Table 2
Factors related to breast cancer
| Risk factors | Age (years) | Years of OCP usage | Frequency of breastfeeding | Frequency of pregnancy |
|---|---|---|---|---|
| Minimum | 32 | 0 | 0 | 0 |
| Maximum | 62 | 16 | 7 | 7 |
| Mean ± SD | 43.7 ± 6.5 | 1.6 ± 3.1 | 2.6 ± 1.3 | 2.7 ± 1.4 |
The mean duration of OCP usage was 1.6 ± 3.1 years with a maximum duration of 16 years. The frequency of breastfeeding and pregnancy was 2.6 ± 1.3 and 2.7 ± 1.4 years, respectively.
Table 3 shows the relationship between different risk factors and sonography results in our subjects.
Table 3
The relationship between risk factors and different mass lesions
The mean age of the cases with ductal carcinoma was more than that of other lesions. Patients with complicated cysts had the minimum duration of pregnancy and breastfeeding, while this duration was greatest in ductal carcinoma. Patients with cancer and ductal ectasia had the longest duration of OCP usage. There was no significant relationship between different types of breast lesions and the prevalence of risk factors in our study.
Table 4 shows the relationship between the degree of breast density and different breast lesions that were detected in sonography; as the table shows, the prevalence of all lesions was higher in cases with dense breast compared to very dense breasts, except for the fibroadenoma, which was detected more in cases with very dense parenchyma. Our study shows that the prevalence of different breast lesions had a significant relationship with the density of the breast.
Discussion
The gold standard for breast cancer screening is mammography; however, sometimes there is a risk that mammography misses some cancers that are more common in dense breasts. With the advances in digital mammography and its increasing accuracy, the risk of error or failure to detect has decreased in women aged less than 50 years, dense breast women, and premenopausal or perimenopausal women [4]. In spite of all the benefits, digital mammography cannot overcome its fundamental restrictions; for example, poor view of non-calcified breast lesions and cancers as a result of overlying dense parenchyma [4,11].
Breast ultrasonography as an adjuvant imaging modality for supplementary assessment of dense-breasted women might help to reveal mass lesions that might be missed by mammography [7].
Although mammographic screening has been successful in decreasing cancer mortality, Madjar et al. showed that mammography-based screening cannot resolve all breast problems. In dense-breasted women, the risk of breast cancer development is increased and the sensitivity of mammography is diminished [12].
In our study, 48.3% of the cases were diagnosed with at least one lesion in their sonography result; although 81% of them were benign lesions, other 19% needed follow-up or biopsy evaluation.
In the study by Min et al., US screening was offered to average-risk and dense-breasted women, resulting in higher detection rates of additional mammographically occult breast cancers [13].
Melnikow et al. suggested that additional screening of dense-breasted women would result in detection of more breast cancers (mostly invasive); however, it might be attributed to higher recurrence rates and additional biopsies [14].
In our study, nine cases were detected as complicated cyst and fibroadenoma and were followed up for 36 months to monitor for any change. In all complicated cyst cases, the size of the lesions was reduced without developing any new lesion, and the fibroadenoma lesions showed no changes during follow-up.
In the study by Melnikow et al. complementary screening of women with dense breasts not only found more breast cancer but also increased the false-positive results. Hence, the effects of complimentary screening on breast cancer outcomes remains unclear [14].
In the study by Scheel et al. a technologist performed US breast screening for women with dense breasts, and after a year small occult breast cancers were detected with a detection rate of 3.2/1000 women screened [15]. In our study, the cancer detection rate was 1 in 207 women screened.
Okello recommends the routine performance of breast ultrasound scan for dense breasts categories 3 and 4. Breast ultrasound scan leads to a considerable breast cancer detection rate among symptomatic dense-breasted women [16].
In the study by Kelikowske et al. the highest sensitivity of mammography was seen in high breast density women aged more than 50 years. The lowest sensitivity was observed in younger women, patients with a family history of breast cancer, or when the time between screenings was about two years. They also mentioned that, because not all dense-breasted women had high interval cancer rates in the study, the breast density should not be considered as the only criterion for deciding whether supplemental imaging is justified. In this study, there was a significant relationship between breast density and prevalence of different malignant and benign lesions [17].
In the study by Min et al. breast density was considered as an independent risk factor for breast cancer [13]. Devoli Disha et al. also considered breast density as a factor that increases the accuracy of sonography in the detection of mass lesions, compared to mammography [18].
Bakkun et al. mentioned the trigger of breast development is ovarian hormones and monthly menstruation regularly develops breast cell proliferation; considering this fact, OCP usage is considered as a risk factor that interferes with monthly menstrual cycles [19].
In different studies, age, OCP usage, breastfeeding duration, frequency of pregnancy, age of first pregnancy and breastfeeding, and family history and previous history of breast cancer were considered as factors that may affect the prevalence of different breast lesions. In our study, there was no significant relationship between these factors and the prevalence of different benign and malignant lesions.


