Introduction
The term polycystic ovarian syndrome (PCOS) represents a heterogeneous and multifaceted entity characterised by hyperandrogenism and/or ovulatory dysfunction. It is also the most common endocrinopathy of women of reproductive age [1], and is associated with an increased cardiovascular risk [2, 3]. According to the Rotterdam criteria (2003) [4] a diagnosis of PCOS can be established when at least two out of three criteria are present (oligo-/anovulation, clinical hyperandrogenism or biochemical hyperandrogenaemia and polycystic ovaries) on condition that other causes of oligo-/anovulation or hyperandrogenism/hyperandrogenaemia (hyperprolactinaemia, Cushing’s syndrome, congenital adrenal hyperplasia, premature ovarian failure, hypothalamic/pituitary disease, etc.), have been ruled out. It is well recognized that a significant proportion of women with PCOS have a propensity towards obesity, dyslipidaemia, insulin resistance, as well as abnormal glucose homeostasis (impaired fasting glucose, impaired glucose tolerance, type 2 diabetes) [5, 6].
Several mechanisms have been implicated in the pathogenesis of insulin resistance in PCOS, including abnormalities in pituitary gonadotropin secretion [7], excessive stimulation of IGF-I receptors, excessive activity of 17α-hydroxylase, an enzyme that regulates conversion of 17-hydroxy-progesterone into androstenedione, as well as diminished synthesis of insulin-like growth factor binding protein 1 (IGF-BP1) [8, 9].
Though some authors claim that insulin resistance may be found in up to 70% of women with PCOS [10], while in a study of Legro et al. [11], based on the data from 254 patients, impaired glucose tolerance was found in 31% of obese and 10.3% of lean women with PCOS, the genuine prevalence of these abnormalities is indeed highly dependent both on the characteristics of the studied population and its ethnic profile [12]. There is however universal agreement that all women with PCOS should be assessed for abnormalities typical for the metabolic syndrome, including lipids and oral glucose tolerance test [13].
Though euglycaemic hyperinsulinaemic clamp technique is considered the gold standard in the assessment of insulin resistance [14], this method is too cumbersome for standard clinical practice. Hence, several other methods have been designed including a homeostatic (HOMA) model based on fasting glucose and fasting insulin (HOMA-IR = [glucose] (mmol/l) × [insulin] (µU/ml)/22.5) [15], or a QUICKI index (QUICKI = 1/[log(I(0)) + log(G(0))], where I(0) denotes fasting insulin, and glucose [G(0) denotes fasting glucose] [16]. There are also methods based an assessment of glucose and insulin during a 75 g glucose tolerance test, such as the insulin resistance index (IRI), originally described by Belfiore et al. [17]. These methods involve an assessment of a dynamic change of glucose and insulin concentrations rather than during a steady (fasting) state. It is, however, not clear whether there is concordance of diagnosis of insulin resistance according to these methods.
The aim of the study was to compare prevalence of insulin resistance in a cohort of women with PCOS by means of the HOMA-IR and IRI methods.
Material and methods
The study involved 137 patients hospitalised in the Department of Endocrinology and Metabolic Diseases of the Medical University of Lodz (Polish Mother’s Memorial Hospital – Research Institute in 2013), i.e. all patients in whom a diagnosis of PCOS was unequivocally established during this year, according to the Rotterdam criteria (2003) [4]. The average age of the patients was (mean ± SD) 25 ±7 years, BMI 27.61 ±7.43 kg/m2. Demographic as well as baseline metabolic characteristics of investigated patients are presented in Table I.
Table I
Descriptive statistics for demographic and clinical data of examined group of 137 patients
Insulin resistance index was calculated from changes of glycaemia and insulinaemia during a 75 g oral glucose tolerance test (OGTT) according to the method described by Belfiore et al. [17]. The IRI was calculated through the formula: ISI(Gly) =2/[1/(INSp × GLYp)] + 1, where INSp and GLYp are the measured insulin and glycaemic areas. In normal subjects ISI(gly) is always around 1, with maximal variations between 0 and 2. This method is based on changes of glycaemia and insulinaemia during OGTT, and correlates well with the euglycaemic hyperinsulinaemic glucose clamp technique [18]. According to some authors the assessment of free fatty acids (FFA) during OGTT is equally effective for the purpose of calculation of the IRI [17]. The cut-off point for this method is quoted as > 1.27 [19].
HOMA-IR index was calculated according to the formula: HOMA-IR = [glucose] (mmol/l) × [insulin] (µU/ml)/22.5 [15].
As there is no universal agreement as to the best cut-off for the HOMA-IR model, we adopted the most commonly used cut-off of 3.8 [20]. There are, however, data based on the analysis of the same, i.e. Spanish population, suggestive that a lower cut-off point (3.46) might be more appropriate for the 90th percentile [21]. Hence, the testing was performed for both the abovementioned cut-off points for HOMA-IR model. The data were subsequently analysed by standard descriptive statistics and by both univariate (Spearman rank correlation) and multivariate models.
Results
Mean HOMA-IR value was 2.72 ±2.24 (median: 2.14, range: 0.33–16.78). The prevalence of insulin resistance in the researched group was 49.6% (68/137) according to IRI, 22.6% (31/137) and 15.8% (21/137) according to HOMA-IR (for the cut-off points of 3.46 and 3.8, respectively). In cases of insulin resistance according to IRI1.27 there was concordance with HOMA-IR3.46 in 83.9% of cases (26/31), while in the case of HOMA-IR3.80, concordance was noted in 85.7% of cases (18/21). On the other hand, the majority of patients found to be insulin-resistant according to IRI (> 1.27) were not insulin resistant according to HOMA-IR (42/68 = 61.7% and 50/68 = 73.5%, for HOMA-IR3.46 and HOMA-IR3.80, respectively). There were only five and three cases of IR according to HOMA-IR with IRI < 1.27 (HOMA-IR3.46 and HOMA-IR3.80 , respectively). Results of this analysis are presented in Tables II and III.
Table II
Comparison of HOMA-IR and insulin resistance (Belfiore) indices for assessment of insulin resistance in women with polycystic ovary syndrome (cut-off for HOMA-IR > 3.46)
Table III
Comparison of HOMA-IR and insulin resistance (Belfiore) indices for assessment of insulin resistance in women with polycystic ovary syndrome (cut-off for HOMA-IR>3.80)
Interestingly, however, among the patients found to be insulin-resistant according to the IRI, those with a concomitant high HOMA-IR index were also found to have higher insulin resistance (Belfiore) indices (1.55 ±0.18 vs. 1.44 ±0.14, p = 0.014, and 1.60 ±0.18 vs. 1.44 ±0.13, p = 0.0008, for HOMA-IR3.46 and HOMA-IR3.80, respectively). Hence, patients with high HOMA-IR (both HOMA-IR3.46 and HOMA-IR3.80), generally tended to be more insulin resistant, with both methods applied.
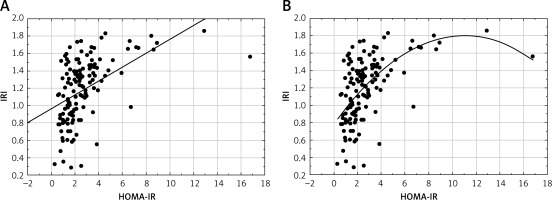
In the next step we assessed the correlation between IRI and HOMA-IR models. The correlation between IRI and HOMA-IR was assessed both in the linear (Figure 1 A) and non-linear models (Figure 1 B). There was a highly significant (p < 0.0001) but only moderate correlation between both models (r = 0.5 and r = 0.57 for a linear and non-linear model, respectively). Furthermore, in this model only 25% of the total variation in HOMA-IR can be explained by the relationship between HOMA-IR and IRI (R 2 = 0.25, p < 0.0001).
Discussion
The issue of insulin resistance in PCOS, though seemingly obvious, is indeed highly problematic, when supposed to be transformed from a theoretical concept into a clinical application. In a seminal paper by Dunaif et al. [22] insulin resistance in PCOS was assessed by means of euglycaemic glucose-clamp technique in obese (n = 19) and non-obese women with PCOS (n = 10) versus obese (n = 11) and non-obese controls (n = 8). The authors concluded that insulin resistance was apparent not in terms of exceeding a predefined cut-off point, but as decreased insulin sensitivity in comparison to BMI-matched non-PCOS peers (expressed as per kilogram total weight or per kilogram fat-free mass or when divided by the steady-state plasma insulin during a euglycaemic clamp). Hence application of any surrogate insulin resistance indices must be viewed with extreme caution.
Furthermore, it must be noted that there is no universal agreement as to the best cut-off point for various insulin-resistance indices. First of all, any cut-off points should be related to particular studied population, as significant ethnic differences have been reported [12]. Secondly, some authors use the 90th percentile to define insulin resistance, for a particular (e.g. HOMA-IR) method [20, 21], while others suggest application of the 75th percentile [19, 23, 24]. As a result of this, significantly lower cut-off points for HOMA-IR have been suggested, e.g. 2.5 [19], or 2.29 [23], or even 2.1 for the population of Krakow (Poland) [24]. Hence, arbitrary application of a pre-defined cut-off point without clear reference to characteristics of a particular studied population, and in the absence of genuine normative data of this population, is indeed problematic, and clearly cannot be validated as scientifically sound. The lack of standardised reference values among surrogate methods of assessment of insulin resistance has been recently raised by some authors [25].
The principal finding of our study is, however, a relatively weak association between the HOMA index derived from fasting glucose and insulin values and a Belfiore index, i.e. one of the methods based on assessment of glucose and insulin concentrations during OGTT. As this method, as well as its variations as described by Matsuda and DeFronzo [18], is principally based on assessment of the area under the curve for glucose and insulin excursions, such a relatively weak correlation (r = 0.5) would also apply to the insulin sensitivity index (ISI)/Matsuda index. As a result of this assessment of insulin resistance by the HOMA-IR index and IRI yields significantly different results according to the method applied. For instance, for a HOMA-IR cut-off point of 3.46, for 68 subjects with raised IRI, only 26 (38.2%) had raised HOMA-IR. This difference was even more striking for a HOMA-IR cut-off of 3.8. The opposite situation, i.e. high HOMA-IR and “normal” IRI, was very uncommon and applied to only 5 (7.25%) and 3 (4.3%) subjects, for HOMA-IR cut-offs of 3.46 and 3.8, respectively. As a result, many more women with PCOS would be diagnosed as insulin resistant with IRI than with HOMA-IR, though those with high HOMA-IR generally tend to have higher IRI indices. This implies that the HOMA-IR index (for a 90th percentile cut-off) also identifies the most insulin-resistant population according to the IRI method.
The discrepancy between HOMA-IR and IRI methods and their relatively weak correlation is not surprising, given that insulin resistance indices derived from fasting glucose and insulin predominantly reflect hepatic rather than peripheral insulin sensitivity [26]. Furthermore, some studies have cast some doubt on the previously assumed excellent correlation of data obtained from these indices and data obtained from a euglycaemic clamp technique, both for fasting glucose and insulin models [27] and for methods based on glucose and insulin during OGTT [28]. In the latter case, some authors raise the issue that indices derived from OGTT could be subjected to many confounders [29]. For instance, significant reductions in β-cell function (where changes in corresponding glucose levels are initially mild) might significantly overestimate insulin sensitivity, while variation in gastric emptying might account for approximately 35% of the variance in peak blood glucose concentrations after ingestion of oral glucose [30], and so this may also seriously alter results obtained from OGTT-based methods.
In conclusion, our study clearly demonstrated that assessment of insulin resistance in women with PCOS is highly method-dependent, and that in a significant percentage of the studied population women might be classified either as “insulin resistant” or “insulin sensitive”, according to the chosen method. Also there is no agreement as to what cut-off points should be used for surrogate measures of insulin resistance. Despite this, some clinicians use surrogate measures of insulin resistance, for instance in order to determine indications for metformin treatment. As the issue of the current place of metformin treatment in PCOS remains debatable, application of surrogate indices of insulin resistance as the sole determinant for the use of insulin-sensitising agents in women with PCOS must be viewed with extreme caution.