Introduction
Oesophageal cancer (OC) is the seventh most common cancer diagnosed worldwide and the sixth most common cause of death among all cancers worldwide. A total of 572,034 new cases of OC (3.2% of all cancers) were diagnosed in 2018. In the same year, 508,585 patients died from this disease (5.3% of all deaths of cancer) [1]. Curative therapy of OC is radical oesophagectomy. Oesophagectomy is the removal of the oesophagus with the tumour along with lymphadenectomy according to oncological principles with subsequent reconstruction of the upper digestive tract utilising tubularised stomach or the large intestine. Mucosectomy is curative only for early stage of the disease (pT1a). Multimodal therapy in the form of neoadjuvant chemoradiotherapy (nCRT) is part of the therapeutic protocol for OC [2]. Even though therapy of OC has improved, the 5-year survival rate after oesophagectomy ranges from 15% to 40% [3]. The main aim of this work is to evaluate long-term outcomes in patients suffering from OC, who underwent hybrid oesophagectomy. In addition, we also evaluated long-term survival in our set of patients with pathological compete response (pCR) after multimodal therapy (neoadjuvant therapy, hybrid esophagectomy) in comparison with patients with partial response (nonCR). The retrospective study of the cohort of patients suffering from oesophageal cancer was approved by the ethics committee of University Hospital Olomouc.
Aim
The main aim of this work is to evaluate long-term outcome in patients suffering from OC, who underwent hybrid oesophagectomy. In addition, we evaluated long-term survival in our set of patients with pathological compete response (pCR) after multimodal therapy (neoadjuvant therapy, hybrid oesophagectomy) in comparison with patients with partial response (nonCR).
Material and methods
Examination
Oesophageal cancer was diagnosed based on endoscopic examination and tumour biopsy. Endosonography and PET/CT were used to determine TNM stage. Tracheobronchoscopy was performed in patients with tumours localised in the proximal and middle part of the oesophagus to rule out tumourous infiltration of the respiratory tract. All patients underwent general internal examination, spirometry, and nutritional evaluation before surgery. Based on the examinations, the patients were classified according to the ASA Physical Status Classification System (ASA).
Neoadjuvant therapy
Neoadjuvant therapy was indicated in cases of advanced clinical stage of the disease (T3-4, N0-2, M0). In patients with tumourous stenosis of the oesophagus (revealed by endoscopy) and in patients suffering from significant dysphagia, a jejunostomy was performed to ensure nutrition prior to oncological therapy. Chemotherapy in the form of combination of 5-fluoruracil and cisplatin was administered. The patients also received radiotherapy – total dose of 45–50.4 Gy and 1.8 Gy/fraction. The disease was restaged after chemoradiotherapy was finished (endoscopy and positron emission tomography/computed tomography (PET/CT) were performed) to assess the effect of neoadjuvant therapy. The patients underwent hybrid esophagectomy 8–10 weeks after nCRT was completed.
Surgery
Oesophagectomy was performed using the hybrid approach, i.e. a combination of minimally invasive and classic open surgery. Resection was performed via minimally invasive approach, and reconstruction was performed by classic open surgery.
Transthoracic oesophagectomy was performed in cases of tumours localised in the thoracic part of the oesophagus. This approach was performed from a right thoracoscopy in modified prone position using selective intubation of the left bronchus with a collapsed right lung. Four incisions for 10 mm ports were made. The camera port was placed in the posterior axillary line in the fifth intercostal space, additional ports were placed in the scapular and mid-axillary line in the seventh intercostal space, and the last auxiliary port was located in the scapular line in the third intercostal space. The oesophagus with the tumour was dissected in its entire transthoracic course, and a harmonic scalpel or LigaSure was used to dissect the mediastinal pleura. The azygos vein was transected by vascular endostapler or with the use of locking Endoclips. Total mediastinal lymphadenectomy was performed during thoracoscopy.
Minimally invasive trans-hiatal laparoscopy was performed in cases of tumours localised in the distal part of the oesophagus and gastroesophageal junction. In this approach the patients were in the supine position with abducted legs and operated under endotracheal anaesthesia. The operation was performed using five incisions with five ports; four 10 mm ports and one 5 mm port. The laparoscope with 30° optics was introduced into the port located 5 cm above umbilicus. Standard mediastinal lymphadenectomy was also performed.
Reconstruction was performed after the transthoracic or trans-hiatal resection phase. We used a left-sided cervical incision in both cases. The oesophagus was completely transected in the deep cervical space. Consequently, the oesophagus with tumour was removed via minilaparotomy. Tubularised gastric conduit was used for transposition in all patients. To construct the gastroplasty, the small curvature of the stomach and the oesophagus were resected using linear staplers; the resection line was always reinforced by suture. The right epiploic artery was preserved to provide nutrition to the conduit. Anastomosis of the gastroplasty and cervical part of the oesophagus was performed using a single-layer continuous suture. Pyloroplasty was also performed in all cases.
The patients were transferred to the intensive care unit after the surgery. Nutrition was administered enterally (nasojejunal probe) and in parenteral form. An X-ray swallow test was performed on day 7 after the surgery. In the case of a satisfactory result and absence of anastomotic fistula, the patients were slowly transferred back to classic enteral nutrition. The patients were followed-up every 3 months for the next 3 years. Follow-up abdominal ultrasonography and chest X-ray were performed every 6 months. PET/CT was performed if necessary. The patients were then followed-up every 6 months during the subsequent 2 years. Follow-up abdominal ultrasonography and chest X-ray were performed once a year.
Statistical analysis
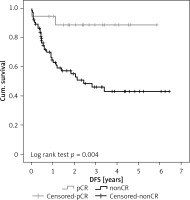
IBM SPSS Statistics version 22 was used for data analysis. Qualitative parameters of cohorts were compared using the χ2 test and Fisher’s exact test, respectively. Student’s t-test was used for comparison of ages. Kaplan-Meier curve was used for comparison of overall survival (OS) and disease-free survival (DFS) (Figure 1). All tests were performed at the level of significance 0.05.
Results
Ninety-four patients (81 males – 86.2% and 13 females – 13.8%) underwent surgery for OC from 2011 to 2015 (Table I). The average age of our cohort was 63.9 years (the youngest patient was 34 years old and the oldest was 81 years old). Thirty-eight (40.4%) patients were younger than 60 years, and the remaining 56 (59.6%) patients were older than 60 years. Adenocarcinoma was present in 57 (60.6%) patients, squamous cell carcinoma (SCC) was present in 34 (36.2%) patients, and other tumour type (small-cell, mucinous carcinoma) was present in 3 (3.2%) patients. In 34 (36.2%) patients the tumour was localised in the upper or middle part of the oesophagus. Sixty (63.8%) patients had the tumour localised in the lower part of the oesophagus or in the gastroesophageal junction. Twenty-one (22.3%) patients were indicated for primary surgery based on TNM classification (T1-2N0M0). Seventy-three (77.7%) patients were indicated for nCRT due to more advanced stage of the disease (T3-4, N0-2, M0). The patients were classified according to American Society of Anaesthesiologists Classification (ASA Class). Forty-nine (52.1%) patients were classified as ASA 3, 43 (45.7%) patients were classified as ASA 2, and 2 (2.1%) patients were classified as ASA 1. Trans-hiatal hybrid oesophagectomy was performed in 83 (88.3%) patients. Transthoracic hybrid oesophagectomy was performed in 11 (11.7%) patients. Trans-hiatal oesophagectomy during the resection phase never required conversion to classic open surgery. Transthoracic minimally invasive approach had to be converted to classic open right thoracotomy due to the size of the tumour and its close anatomical relation to the tracheal bifurcation in 3 cases. Severe intraoperative complications did not occur.
Table I
Cohort of patients who underwent hybrid oesophagectomy for oesophageal cancer from 2011 to 2015
Blood loss ranged from 100 ml to 3500 ml, with an average of 555 ml. Blood transfusion had to be administered to 52 (55.3%) patients either during or after the surgery. Duration of surgery ranged from 130 min to 345 min, with an average of 222 min. Lymphadenectomy was performed in all patients. On average, 11.7 lymph nodes were removed (minimum 5 and maximum 28). Respiratory complications predominated in the postoperative period. Severe respiratory complications (acute respiratory distress syndrome (ARDS) and respiratory failure) occurred in 11 (11.7%) patients. Mild respiratory complications (pneumonia, pleural effusion, postoperative pneumothorax, and atelectasis) occurred in 35 (37.2%) patients. Cardiac complications occurred in 8 (8.5%) patients. Anastomotic fistula developed in 9 (9.6%) patients. Three fistulas required a combination of surgical drainage of the deep cervical space and antibiotic therapy. Six fistulas were managed conservatively – the fistula was small in all cases and follow-up swallow test was physiological. Paresis of the recurrent laryngeal nerve developed in 9 (9.6%) patients. Oedema in the region of surgery was the cause in 5 cases. The pareses improved after some time. An incisional hernia developed in 3 (3.1%) patients. It was treated surgically by mesh implantation. The shortest hospitalisation lasted 11 days and the longest was 119 days, with an average of 22.5 days.
Two patients died during the 30-day postoperative period due to severe respiratory complications, and 2 patients died during the 90-day postoperative period due to respiratory complications and multi-organ dysfunction syndrome that developed due to sepsis resulting from massive dehiscence of the anastomosis and mediastinitis. The complications were classified according to the Clavien-Dindo Classification – 10 (10.6%) patients showed grade I, 47 (50%) patients showed grade II, 17 (18%) patients showed grade IIIa, 7 (7.4%) patients showed grade IIIb, 5 (5.3%) patients showed grade IVa, 1 (1%) patient showed grade IVb, and 4 (4.2%) patients showed grade V.
Eighteen (24.7%) patients presented with pathological complete response (pCR) to neoadjuvant therapy in the resected specimen, i.e. no tumour was present. The remaining 55 (75.3%) patients presented with signs of malignant tumour in the resected specimen (nonCR). Statistical analysis of our results revealed the following: We proved a statistically significant association between pCR and age. Patients with pCR were significantly younger – they were mostly below 60 years of age (p = 0.017), Table II. Patients with pCR also showed significantly longer DFS, 3.4 years vs. 2.5 years (p = 0.004), Figure 1. A statistically significant difference was not proven for different sex, tumour type, tumour localisation, and OS after the surgery.
Table II
Comparison of patients with pCR and without pCR. Patients with pCR were significantly younger – below 60 years of age (p = 0.017). Statistically significant difference in sex, tumour type, its localisation, and the number of deaths was not proven
Patients with adenocarcinoma had the tumour significantly more often localised in the distal part of the oesophagus (p < 0.0001), Table III. A statistically significant difference was not proven for different sex, pCR, OS, and DFS. Men and women did not show a statistically significant difference in tumour type, localisation, pCR, and number of deaths. Women were significantly older than men (p = 0.041). No statistically significant relationship between sex and OS (p = 0.135) or DFS (p = 0.616) was proven.
Table III
Comparison of results according to tumour type. Patients suffering from adenocarcinoma showed significantly higher occurrence of tumour in the distal part of the oesophagus (p < 0.0001). Statistically significant difference in sex, age, presence of pCR, and number of deaths was not proven
Discussion
The only curative therapy for OC is oesophagectomy. It can be performed using classic open surgery, hybrid technique (combination of classic open surgery and minimally invasive approach), or completely using the minimally invasive approach. The choice of a particular approach should be based on preoperative staging of the disease, the size of the tumour, its histological characteristics, the patient´s comorbidities, the anatomical and physiological status of the patient, and the experience of the surgeon [4]. Minimally invasive techniques have been preferred over the past decade. Less surgical trauma, fewer respiratory complications, and shorter recovery period are reported in cases of minimally invasive oesophagectomy [5, 6]. Hybrid oesophagectomy is also associated with better postoperative outcomes compared to the open approach, especially concerning pulmonary complications. For long-terms outcomes, the hybrid approach showed similar, or even better, overall survival than the open approach [7].
It is important to preserve surgical radicality with proper lymphadenectomy when using any of the approaches [8]. Tachibana et al. recommend removing at least 12 lymph nodes [9]. The number of metastatic lymph nodes (< 3 vs. ≥ 3) was an independent prognostic factor for OS (p = 0.02), as demonstrated by Gulben et al.; the 5-year OS rates in patients with < 3 MlNs and ≥ 3 MlNs were 17% (median: 34 months) and 5% (median: 18 months), respectively [10].
A multicentric randomised controlled trial (total number of patients 207) showed that the use of hybrid minimally invasive oesophagectomy results in a decrease of severe intraoperative and postoperative complications (especially respiratory) by 77% when compared to classic open oesophagectomy. Overall survival and disease-free survival were the same or longer in patients after minimally invasive surgery compared to patients having undergone the open procedure over a period of 3 years [11]. We prefer the hybrid oesophagectomy at our workplace. Resection is performed by minimally invasive approach, and reconstruction is done via classic open surgery. The surgery is performed via trans-hiatal approach for tumours located in the aboral part of the oesophagus. Thus, incision of the thoracic wall is not necessary. Reconstruction is performed using the tubularised gastric conduit. Anastomosis to the remaining cervical oesophagus is then performed in the deep cervical space. Coloplasty is performed when the stomach cannot be used as transponate (due to malignant infiltration of oesophagus or previous gastric surgery).
To achieve optimal short- and long-term results following surgery for oesophageal cancer, oesophageal surgeons are in agreement that these procedures should be performed in specialised high-volume centres. Minimally invasive procedures should be performed by experienced surgeons in centres specialising in minimally invasive procedures.
In the past, oesophagectomy was associated with a high perioperative morbidity and mortality. Nowadays, perioperative morbidity ranges from 20% to 50% [12, 13]. Respiratory complications are most serious and occur in 19–44% of all cases [14]. These are dangerous especially for old patients, smokers, patients suffering from malnutrition, patients with low forced expiratory volume in 1 s (FEV1), patients who have received neoadjuvant therapy, and patients who develop anastomotic fistula [12, 13]. Our cohort of patients also experienced respiratory complications to be most common, which corresponds with the experience of other research teams. Respiratory complications developed in 48.9% of cases (11.7% were severe and 37.2% were mild). Necrosis of the transponate is most dangerous from a surgical point of view. However, this complication is rare – 0.5% [15]. According to the literature, dehiscence of the anastomosis occurs in 5–20% of all cases [16].
Perioperative mortality is defined as death during surgery or in the 30 following days. Postoperative mortality significantly decreased when compared to previous decades, especially in high-volume centres where it ranges from 1% to 5% [17, 18]. A study of the Society of Thoracic Surgeons General Thoracic Surgery Database (STS GTSD) reported perioperative mortality of 3.1% in a cohort of 4321 patients who underwent oesophagectomy [19]. Also, a study of American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) which analysed a cohort of 1032 patients who underwent oesophagectomy reported a 3.0% mortality [20].
Even though treatment of oesophageal tumours has improved, the 5-year survival rate still ranges from 15% to 40% [3]. The randomised study Chemo Radiotherapy for Oesophageal cancer followed by Surgery Study (CROSS) reported an increase in 5-year survival rate by 14% in patients who received nCRT with consequent surgery when compared to patients who underwent only surgery. Moreover, 29% of patients who received nCRT showed pCR (49% of SCC and 23% of adenocarcinoma) [21]. Similar results were observed in our study; nearly 25% of our patients had pCR in the definite histological finding.
Chirieac et al. studied a cohort of 235 patients either with invasive SCC or with adenocarcinoma of the oesophagus or oesophagogastric junction. Residual carcinoma in the resected specimen was divided into four categories: no residual carcinoma (stage 0), 1–10% of residual carcinoma (stage I), 11–50% of residual carcinoma (stage II), and > 50% of residual carcinoma (stage III). The DFS rate was significantly higher in patients with stage 0 (60% after 5 years) when compared to patients with more advanced stages. Patients with stage I and stage II did not show a statistically significant difference in DFS (p = 0.67). The OS rate of patients in stage 0 was 65% after 5 years. Patients in other stages with residual carcinoma showed an OS rate of 29% (p = 0.003) after 5 years. Chirieac also points out that preoperative results of examinations as well as clinical stage of the disease did not have any influence on DFS and OS. He better predicts the OS based on findings of residual carcinoma in the resected oesophagus or gastroesophageal junction. The patients who presented with no residual carcinoma in the resected specimen showed significantly better results [22]. In our cohort we also had patients who showed pathological complete response to neoadjuvant chemoradiotherapy, i.e. no residual carcinoma was revealed in the resected specimen. Pathological complete response was present in 24.7% of cases. There was no statistically significant difference in occurrence of pCR in patients with adenocarcinoma and SCC.
Darton et al. and Meluch et al. also report similar results [23, 24]. Lin et al. showed in their study that pCR is a positive prognostic factor of better outcome in patients with locally advanced tumour, who received nCRT and consequently underwent oesophagectomy [25]. Alnaji et al. analysed a cohort of patients with oesophageal adenocarcinoma, who received nCRT and consequently underwent oesophagectomy. Nineteen percent of patients showed pathological complete response. Overall 3-year survival was 86% in patients who showed pCR. On the other hand, overall 3-year survival of patients who showed nonCR was 48%. The disease-free survival rate was 80% in patients with pCR and 39% in patients with nonCR. Thus, pCR can be considered as an independent prognostic factor of better OS and DFS [26]. In our cohort we proved significantly longer DFS when compared to the aforementioned studies. However, we did not prove longer OS.
Conclusions
Combined oesophagectomy with neoadjuvant chemoradiotherapy results in better long-term outcome in patients suffering from oesophageal cancer. In our set of patients, who underwent hybrid oesophagectomy, satisfactory short-term and especially long-term results of surgical treatment for oesophageal cancer were observed. Further studies are necessary to support the results reported in this manuscript.