Introduction
Immunoglobulin A vasculitis (IgAV), also referred to as Henoch-Schönlein purpura, is an immune-mediated vasculitis associated with immunoglobulin A (IgA) deposition. It is characterized by leukocytoclastic vasculitis accompanied by IgA immune complexes within affected organs [1, 2]. The main clinical symptoms of IgAV include cutaneous purpura, abdominal pain, hemorrhagic gastroenteritis, arthritis and kidney damage [3]. Among them, IgAV nephritis (IgAVN) is potentially the most severe complication, which usually manifests as proteinuria, hematuria, and even renal failure [4]. IgAVN occurs more frequently in children than in adults and it was listed as the most common secondary glomerular disease in children [5]. However, adult IgAVN was usually observed to be more severe than the disease in children because it tended to result in a poor prognosis [6].
Although the complex pathogenesis of IgAVN has not been fully elucidated yet, recent studies revealed that immune abnormalities played a significant role in the pathogenesis of IgAVN [7]. CD4+ T cells are important cells involved in the immune response. Regulatory T-cells (Tregs) and Th17 cells, which belong to CD4+ T cell subsets, maintain the body’s immune balance. Tregs are responsible for the maintenance of self-tolerance, thus inhibiting autoimmunity, whereas pro-inflammatory Th17-cells contribute to the induction and propagation of inflammation [8]. Tregs, marked by Foxp 3, were reported to secrete IL-10 to prevent inflammatory and autoimmune pathologies [9, 10]. Th17 expressed high levels of IL-17A (commonly referred to as IL-17), which is recognized as one of the key effector cytokines of Th17 cells to promote an inflammatory response [8, 11]. An imbalance of the Th17/Treg cell axis has been observed in a variety of autoimmune nephropathies, such as IgA nephropathy, minimal change nephrotic syndrome and lupus nephritis [12-14]. An imbalance of the Th17/Treg cell axis also contributed to the pathogenesis of IgAV in children [15].
However, there are few studies to report the imbalance of the Th17/Treg cell axis in adult IgAVN. Thus, we examined the Th17/Treg cell axis in peripheral blood of adult patients with IgAVN in the present study. Recently, red blood cell distribution width (RDW) was also reported to be a novel inflammatory marker in several kinds of inflammatory diseases [16-18]. In the present study, we also examined the relationship between the ratio of Th17/Treg cells and of adult IgAVN patients, aiming to provide some new immunological viewpoints for the pathogenesis of adult IgAVN.
Material and methods
Reagent
PerCP Mouse Anti-Human CD4 (Cat# 347324), FITC Mouse Anti-Human CD25 (Cat#555431), PE Mouse anti-Human Foxp 3 (Cat# 560852), PE Mouse anti-Human IL-17A (Cat# 560486), Permeabilizing Solution 2 (Cat# 340973), Lysing Solution (10X) (Cat# 349202), and Protein Transport Inhibitor (Cat# 51-2092KZ) were purchased from BD Biosciences, USA. Red Blood Cell Lysis Buffer (Cat# 420301) was obtained from Biolegend, Inc. TRIzol solution (Cat# 15596026) was purchased from Ambon, USA. SYBR Green PCR kit (Cat# KM4101) and cDNA first strand synthesis kit (Cat# 639505) were provided by KAPA Biosystems, USA and Takara Bio, Japan respectively. DNase I (Cat# AM2295) was obtained from Fermentas, Lithuania. PCR primers were synthesized by Wuhan Tianyi Huiyuan Biotechnology Co., Ltd. All other reagents were of the highest quality available from commercial vendors.
Subjects
This study was approved by the Ethics Committee of Hubei Provincial Hospital of Traditional Chinese Medicine and in accordance with the Declaration of Helsinki (No. hbzy2019-c37-01). The study was also registered in the China Clinical Trial Registration Center (No. CHiCTR1900025155). All IgAVN patients (8 males and 4 females with the age of 39.25 ±13.13) and healthy subjects (6 males and 7 females with the age of 32.85 ±9.45) provided written informed consent before data collection. Healthy subjects were recruited from the Health Assessment Center in our hospital. They had neither a history of immunological disorders nor a history of taking immunosuppressive drugs. The IgAVN diagnosis was made according to Clinical Guidelines-a division of nephrology edited by the Chinese Medical Association in 2011 [19].No patient had received glucocorticoids or other immunosuppressive drugs for the past 3 months before enrolling in the study. Basic characteristics of healthy subjects and adult IgAVN patients are provided in Tables 1 and 2 respectively.
Table 1
Characteristics and laboratory data of healthy subjects
Table 2
Characteristics and laboratory data of adult IgAVN patients
[i] HSPN – Henoch-Schönlein purpura nephritis, WBC – white blood cells, NEU – neutrocytes, LYM – lymphocytes, MONO – monocytes, PLT – platelets, RDW – red blood cell distribution width, HGB – hemoglobin, ALB – albumin, U-PRO – urinary protein, U-BLD – urinary blood, BLO – urinary red blood cells, eGFR – estimated glomerular filtration rate
Analysis of Th17 cells in peripheral blood
Fresh blood samples were treated according to the instructions of Human Th1/Th2/Th17 Phenotyping Kit (BD Pharmingen, BD Biosciences, USA). After that, cells were fixed and permeabilized, and PerCP Mouse Anti-Human CD4 and PE Mouse anti-Human IL-17A antibodies were added to incubate with cells at room temperature for 30 minutes according to the manufacturer’s instructions. Then cells were washed, resuspended and measured on the machine. Isotype controls were used to confirm antibody specificity. Data were analyzed by a FACSCalibur flow cytometer equipped with CellQuest software (Becton Dickinson, USA).
Analysis of Treg cells in peripheral blood
Cells were incubated with PerCP Mouse Anti-Human CD4 and FITC Mouse Anti-Human CD25 at room temperature for 20 minutes. Then, 2 ml of Lysing Solution (1X) were added, followed by 10 minutes’ incubation at room temperature in the dark. Cells continued to be stained with PE Mouse anti-Human Foxp 3 antibody for 20 minutes in the dark. After washing and resuspending, cells were measured on the machine. Data were analyzed by a FACSCalibur flow cytometer equipped with CellQuest software.
Detection of Foxp3 mRNA in peripheral blood
Frozen blood samples were treated by Red Blood Cell Lysis Buffer. Then, 1.0 ml of TRIzol solution was added to a centrifuge tube of 1 × 106 cells to extract total RNA, and cDNA synthesis was carried out according to the manufacturer’s instructions. The specific sequence of Foxp3 was set up as 5'-ACCAAGGCTTCATCTGTG-3', 5'-CTCTGGGAATGTGCTGTT-3', product size 216 bp. β-actin was used to as a control, 5'-ACACTGTGCCCATCTACG-3', 5'-TGTCACGCACGATTTCC-3', product size 153 bp. Real-time PCR was performed according to the description of the SYBR Green PCR kit. The reaction procedure was set up as follows: 95°C, 3 min; 95°C, 5 s; 56°C, 10 s; 72°C, 25 s; 39 cycles; 65°C, 5 s; 95°C, 50 s. After the amplification, the data were analyzed by the Fluorescent Quantitative PCR Amplification Instrument (CFX-Connect 96, Bio-Rad, USA).
Quantification of IL-17 and IL-10 in serum
Concentrations of IL-17 and IL-10 in the serum were measured by enzyme-linked immunosorbent assay according to the manufacturer’s instructions (IL-17 Cat#HM10198, IL-10 Cat#HM10203, Bioswamp, China). Data were collected by a microplate reader (352 type, Labsystems Multiskan MS, Finland).
Statistical analysis
Differences of values between healthy subjects and adult IgAVN were analyzed with the Mann-Whitney test. The relationship between red blood cell distribution width and ratio of Th17/Treg in adult IgAVN patients was analyzed by Spearman correlation analysis. These analyses were performed with GraphPad PRISM 8.0.1 (GraphPad Software Inc., San Diego, CA). In each case, two-sided p values < 0.05 were considered to be significant.
Results
Th17 cell and Treg cell frequencies in peripheral blood of healthy subjects and adult IgAVN patients
As shown in Figure 1B, CD4+ IL17A+ was recognized as Th17 cells. The percentages of CD4+ Th17+ cells in peripheral blood of healthy subjects and adult IgAVN patients were 2.65 ±1.55% and 4.37 ±1.68% respectively (Fig. 1A). The frequency of CD4+ Th17+ cells of adult IgAVN patients was significantly higher than that of healthy subjects (p = 0.0019).
Fig. 1
Th17 cell and Treg cell frequencies in peripheral blood of healthy subjects and adult IgAVN patients. B, D) The typical histograms of Th17 cell and Treg cell frequencies in peripheral blood of healthy subjects and adult IgAVN patients respectively. Statistical results are summarized in A (Th17 cells) and C (Treg cells). E) The ratio of Th17 and Treg cells in peripheral blood of healthy subjects and adult IgAVN patients

As shown in Figure 1D, CD4+ CD25+ Foxp3+ was recognized as Treg cells. The percentages of Treg cells in peripheral blood of healthy subjects and adult IgAVN patients were 6.44 ±2.90% and 3.91 ±1.94% respectively (Fig. 1C). The frequency of Treg cells of adult IgAVN patients was significantly lower than that of healthy subjects (p = 0.0135).
Therefore, an imbalance of Th17/Treg was observed in adult IgAVN patients (Fig. 1E). The ratio of Th17/Treg in adult IgAVN patients was significantly higher than that of healthy subjects (p = 0.0030).
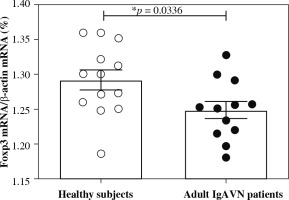
Foxp3 mRNA expression in peripheral blood of healthy subjects and adult IgAVN patients
Next, we continued to examine the Foxp3 mRNA expression in peripheral blood of healthy subjects and adult IgAVN patients. As shown in Figure 2, the Foxp3 mRNA expression of adult IgAVN patients was significantly lower than that of healthy subjects (p = 0.0336).
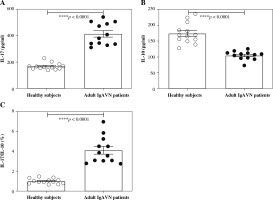
IL-17 and IL-10 in peripheral blood serum of healthy subjects and adult IgAVN patients
We also detected the IL-17 and IL-10 in peripheral blood serum of healthy subjects and adult IgAVN patients using ELISA. The data are provided in Figure 3. The concentration of IL-17 in peripheral blood serum of adult IgAVN patients (413.29 ±84.30 pg/ml) was significantly higher than that of healthy subjects (167.09 ±24.97 pg/ml) with a p value lower than 0.0001 (Fig. 3A). However, the concentration of IL-10 in peripheral blood serum of adult IgAVN patients (104.53 ±13.72 pg/ml) was significantly lower than that of healthy subjects (174.21 ±32.18 pg/ml) with a p value lower than 0.0001 (Fig. 3B). Thus, there was a significant difference in the ratio of IL-17/IL-10 between healthy subjects and adult IgAVN patients (p < 0.0001, Fig. 3C).
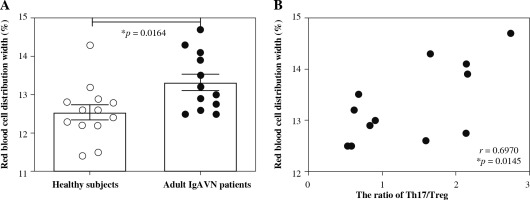
Relationship between RDW and ratio of Th17/Treg in adult IgAVN patients
The significant differences of RDW between healthy subjects and adult IgAVN patients, as shown in Figure 4A, reminded us to examine the relationship between RDW and the ratio of Th17/Treg in adult IgAVN patients. There was a significant correlation between RDW and the ratio of Th17/Treg in adult IgAVN patients in Spearman correlation analysis (r = 0.6970, p = 0.0145, Fig. 4B).
Fig. 4
Relationship between red blood cell distribution width and ratio of Th17/Treg in adult IgAVN patients. A) The significant differences in red blood cell distribution width between healthy subjects and adult IgAVN patients. B) The relationship between red blood cell distribution width and ratio of Th17/Treg in adult IgAVN patients
Discussion
A national cross-sectional survey in China reported that IgAVN was listed as one of the most common secondary glomerular diseases among children [20]. Most pediatric IgAVN patients present with hematuria and/or low-grade proteinuria, and their prognosis is generally satisfactory [7]. Nearly 5-15% of pediatric IgAVN patients were reported to progress to chronic renal failure [21]. However, this rate was approximately 30% in adult IgAVN patients [22]. The present study was designed to investigate the possible immunological pathogenesis of adult IgAVN with a focus on the Th17/Treg cell axis. According to our best knowledge, it is the first report of imbalanced Th17/Treg in peripheral blood of adult IgAVN patients. Moreover, the significant correlation between RDW and the ratio of Th17/Treg in adult IgAVN patients seems to be a new finding.
CD4+ Th17+ cells and their effector cytokines mediate the crucial crosstalk between the immune system and tissues, and are involved in the pathogenesis of many autoimmune diseases [23]. In Figure 1A, we also observed that the frequency of CD4+ Th17+ cells of adult IgAVN patients was significantly higher than that of healthy subjects (p = 0.0019). IL-17 was one of the key effector cytokines of Th17 cells [8, 11]. It was reasonable to obtain a higher concentration of IL-17 in the serum of adult IgAVN patients in Figure 3A (p < 0.0001). Treg cells, most of which are produced by the normal thymus as a functionally mature T-cell subpopulation, play key roles in the maintenance of immunologic self-tolerance and negative control of a variety of physiological and pathological immune responses [24]. We also observed that the frequency of Treg cells of adult IgAVN patients was significantly lower than that of healthy subjects (p = 0.0135, Fig. 1C). Natural Tregs specifically express Foxp3, a transcription factor that plays a critical role in their development and function [24]. As shown in Figure 2, the Foxp3 mRNA expression of adult IgAVN patients was significantly lower than that of healthy subjects (p = 0.0336), which was consistent with the above result. Consequently, as an important mediator of Treg suppression, the concentration of IL-10 in the serum of adult IgAVN patients was significantly lower than that of healthy subjects, as shown in Figure 3B [25]. All these findings strongly suggested that imbalanced Th17/Treg existed in adult IgAVN patients (Figs. 1E and 3C).
Red blood cell distribution width is a simple and inexpensive parameter, which reflects the degree of heterogeneity of erythrocyte volume (conventionally known as anisocytosis), and is traditionally used in laboratory hematology for differential diagnosis of anemias [26]. Recent studies revealed that RDW levels in IgAVN were significantly higher than those in IgAV without nephritis, and RDW would be useful in predicting the presence of crescents on histopathology [27, 28]. Our observation was consistent with these studies (Fig. 4A). RDW was also reported to be a novel inflammatory marker in several kinds of inflammatory diseases, such as septic shock, inflammatory bowel disease, and acute appendicitis [16-18]. Distortion of the Th17/Treg balance favoring the pro-inflammatory Th17 side was suspected to contribute to exacerbation of autoimmune disorders [8]. Therefore, we were reminded to examine the correlation of RDW and the ratio of Th17/Treg in adult IgAVN patients (Fig. 4B). A significant correlation between RDW and the ratio of Th17/Treg in adult IgAVN patients was observed in the present study (r = 0.6970, p = 0.0145). However, how RDW affects the Th17/Treg cell axis in adult IgAVN patients needs to be addressed in the future.
In the present study, we found imbalanced Th17/Treg in adult IgAVN. This imbalance of the Th17/Treg cell axis was also observed in IgA nephropathy patients [13]. On the other hand, both IgAVN and IgA nephropathy are characterized by circulating immune complexes and their mesangial deposition induced by galactose-deficient IgA1 (Gd-IgA1) [7, 29]. The above information suggests that regulation of the Th17/Treg cell axis might be helpful to reduce the production of circulating immune complexes for both IgAVN and IgA nephropathy.
Numerous studies demonstrated that adult IgAVN patients have more severe renal histopathological changes than pediatric patients [22, 30, 31]. Although Gd-IgA1 was identified to be involved in the pathogenesis of IgAVN, no significant differences were observed between the Gd-IgA1 level in adult and child IgAVN patients [32, 33]. Until now, the difference in the immunological pathogenesis of adult and child IgAVN is still unclear. The imbalance of the Th17/Treg cell axis plays a significant role in the pathogenesis of a variety of autoimmune nephropathies [12-14]. It is certain that Th17/Treg cell axis imbalance exists in both adult and child IgAVN as reported in the present and previous studies [15]. However, whether the imbalance of the Th17/Treg cell axis is more severe in adult IgAVN than pediatric patients should continue to be investigated in the future.