Introduction
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), encompasses many chronic, long-life, multifactorial polygenetic diseases [1]. Patients with IBD may be at increased risk of any infections due to frequent hospitalizations, numerous blood sample collection, and blood transfusions [2]. Long-term treatment for IBD involves the use of anti-inflammatory agents and immunosuppressive medications, such as steroids, antimetabolites, and biologic therapies. The immunosuppressive nature of the treatment as well as disease-associated malnutrition could compromise the efficacy and safety of vaccination [2].
For all these reasons, prevention of infections, including hepatitis B, through vaccination is in the best interest of patients with IBD [2]. There are several hepatitis B immunization regimens, all of which have been studied in healthy populations. The most recommended schedule for the hepatitis B vaccine consists of three doses given at 0, 1, and 6 months [3]. This schedule is recommended for immunization of all infants born at term worldwide, including Poland. Vaccination with the hepatitis B vaccine induces protective levels of anti-hepatitis B surface antibodies (anti-HBs ≥ 10 mIU/ml) in the majority (approximately 95%) of healthy infants [4]. The cut-off of 10 mlU/ml arose from technical features of the test preventing the detection of fewer than 10 units. Currently, fewer units are also detectable [5]; however, an antibody level below 10 mIU/ml is still classified as a non-response to the vaccine. In 2006, it was shown that immunocompetent individuals immunized with 3 doses of the hepatitis B vaccine and with post-vaccination antibody levels above 10 mlU/ml did not need a booster dose of vaccine even if they have undetectable antibody levels later in life [6]. This protection is a result of the immune memory (the presence of memory cells) that induces antibody production in the event of contact with hepatitis B virus (HBV) antigens [7]. It has been demonstrated that a booster dose of the vaccine (contact with HBV antigens) restores the immune response in patients with IBD, who have lost their seroprotection, and this booster was thus included in the recommended schedule for these cases [8]. However, questions regarding what level of anti-HBs is protective and what the advisable frequency of anti-HBs monitoring is in immunosuppressed patients are still open to a debate.
To date, based on the results of a meta-analysis by Jiang et al., the immunogenicity of hepatitis B vaccine in adult patients with IBD was determined to be 61% [9]. In children with IBD, in a small number of studies, immunity to hepatitis B varied from 28% to 70.2% [10-12], but the children were not vaccinated during infancy in any of these studies.
According to the European Crohn’s and Colitis Organization, vaccination against HBV is strongly recommended in all seronegative patients with IBD. However, immunogenicity of hepatitis B vaccination may be impaired in IBD, both by the disease itself and by immunosuppressive therapy. In adult IBD patients, the administrations of accelerated double-dose of the vaccine followed by double-dose re-vaccination (if no adequate response is achieved) has demonstrated a better efficacy than the standard schedule (60% to 70% efficacy) [13]. In children with IBD, a single booster dose of hepatitis B vaccine has demonstrated to restore immune response in pediatric patients, who had lost seroprotection, and is the recommended schedule in these cases [11].
The aim of this study was to evaluate the immunogenicity of hepatitis B vaccine in pediatric patients with IBD, who were fully (3 doses) vaccinated against HBV in infancy. We also assessed the immune response to booster doses of hepatitis B vaccine in non-seroprotected patients.
Material and methods
This study was conducted in three university-affiliated hospitals for children in Poland, Warsaw, Poznan, and Wroclaw, between January 2015 and December 2017.
Study participants
The study population consisted of 1- to 18-year-old children with IBD (CD and UC), each with a documented full (3 doses) series of hepatitis B vaccination in infancy. Both hospitalized and ambulatory patients were invited to participate in the study. The diagnosis of CD and UC was based on clinical signs and symptoms as well as endoscopic, histological, and radiological results according to the Porto criteria. The severity of CD and UC was evaluated using the pediatric Crohn’s disease activity index (PCDAI) and the pediatric ulcerative colitis activity index (PUCAI), respectively; PCDAI score ≤ 10 for CD and PUCAI score < 10 for UC were defined as a remission.
It has been previously described that patients with IBD on combination therapy, i.e. anti-TNF-α agents and immunomodulators given concomitantly, has a significantly lower response rate than those on monotherapy [9, 13]. Therefore, at the start of our study, patients were stratified into the following groups: 1. Patients with IBD who were not receiving any immunosuppressive therapy, i.e., patients receiving 5-ASA (at any dose); 2. Patients who were receiving immunomodulatory therapy such as 2.5-3 mg/kg/day azathioprine (AZA) or 1.5 mg/kg/day 6-mercaptopurine (6-MP) 12 weeks or more, > 0.4 mg/kg/week methotrexate or any dose of cyclosporine; patients on steroids at a dose of ≥ 20 mg/day for 2 weeks or more; 3. Patients receiving biologic therapy such as infliximab or adalimumab (at any doses and schedules); 4. Patients who were treated with combination therapy with immunomodulators and biologics concomitantly.
Intervention
For all patients, demographic data and medical history were collected, and physical examinations were performed. Child’s health record books of all included patients were reviewed. If any doubts occurred, their individual immunization cards were verified. Disease activity was assessed with the PCDAI for CD and the PUCAI for UC. HBV infection was excluded based on a negative HBs and HBc antigen tests. In all children, quantitative serum anti-HBs was measured by chemiluminescent microparticle immunoassay (CMIA) (Abbott Laboratories, Abbott Park, IL, USA). Anti-HB levels ≥ 10 mIU/ml were considered an adequate immune response. Anti-HBs levels ≥ 100 mIU/ml were defined as an effective immune response to the hepatitis B vaccine.
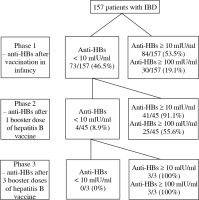
The study consisted of 3 phases (Fig. 1). In phase 1, anti-HBs titer was measured in all children. In phase 2, only children with anti-HBs < 10 mlU/ml were vaccinated with one dose of hepatitis B vaccine, and the anti-HBs titer was measured after 8 weeks. Only patients with anti-HBs titer < 10 mIU/ml were included to the phase 3 of the study; they received two additional doses of hepatitis B vaccine, and the anti-HBs titer was measured 8 weeks after the last dose of the vaccine. The hepatitis B vaccine (Engerix B®, GlaxoSmithKline, Rixensart, Belgium) was given at doses of 10 µg and 20 µg for children < 16 years and ≥ 16 years, respectively. The vaccine was administered intramuscularly into the deltoid muscle. Patients were observed by the investigators for immediate reactions for 30 minutes after vaccination.
Outcomes
The primary outcome measure was the adequate immune response rate in the four study groups. The secondary outcome measure was the number of patients with an effective immune response. Outcome measures were assessed at study initiation and 8 weeks after the first and last dose of the booster vaccine. The influence of treatment type in the four study groups on immune response was also assessed.
Sample size
Sample size was calculated based on a previous study assessing the immune response against the hepatitis B vaccine in children with IBD [13]. To detect a difference of 25% between patients with anti-HB levels ≥ 10 ml/IU on immunosuppressive therapy and those on non-immunosuppressive therapy, with an alpha level of 0.05 and beta level of 0.1, 75 children were required in each group. To account for an expected dropout rate of 20%, a total sample of 180 children was required.
Statistical analysis
Continuous variables were tested with Shapiro-Wilk test of normality. Data were then expressed as the median and interquartile range (IQR). Mann-Whitney U test was used to test for differences between two numeric variables, and χ2 test was used to compare proportions. Data analysis was conducted by using Statistica 12 (Statsoft, Oklahoma, USA).
Ethical consideration
Approval from the Clinical Research Ethics Committee of the Medical University of Warsaw, Poland, was obtained before the study started (KB/165/2014). All parents and children ≥ 16 years old have signed informed consent forms before participation in the study.
Results
In total, 157 patients with IBD (92 patients with CD and 65 patients with UC) were included. The baseline characteristics of study group are shown in Table 1. Figure 1 presents the number of patients with anti-HBs levels ≥ 10 mIU/ml and anti-HBs levels ≥ 100 mIU/ml in the 3 phases of the study.
Table 1
Baseline characteristics of the study participants
Phase 1: The immune response against HBV after hepatitis B vaccination in infancy
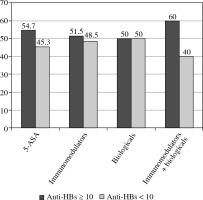
Of 157 patients, 84 (53.5%) had anti-HBs levels ≥ 10 mIU/ml (Fig. 1). The type of therapy was not associated with the hepatitis B vaccine response rate (p = 0.9), as shown in Figure 2. Higher rates of anti-HBs levels ≥ 10 mIU/ml were observed in patients treated with 5-ASA, immunomodulators without and with biologicals; however, the differences were not statistically significant (p = 0.4, 0.7, and 0.9, respectively). We did not find any statistically significant differences in patient age, disease type, duration, or activity between patients who responded and those who did not respond to the hepatitis B vaccine (Table 2).
Table 2
Patients who responded and did not respond to hepatitis B vaccination
Phases 2 and 3: The immune response against HBV after 1 and 3 doses of anti-HBV booster vaccination
After booster vaccination (1 dose of the vaccine) against HBV, 41/45 (91.1%) patients had an anamnestic response (Fig. 1). Of those patients who did not respond, all were treated with immunomodulators in monotherapy (p = 0.02). All the patients, who did not respond to the booster dose of vaccine (3/45), received additional 2 doses of hepatitis B vaccine, and all of them (3/3) achieved protective anti-HBs levels (Fig. 1). One patient missed a scheduled follow-up visit.
Discussion
In this prospective study, we found that only 53.5% of children and adolescents with IBD, who received the full (3 doses) series of hepatitis B vaccines in infancy were protected against HBV infection. However, after 3 additional doses of hepatitis B vaccine, all patients were considered protected against HBV infection. Our study is the first “real-life study”, because we analyzed a group of children with IBD who were vaccinated against hepatitis B in infancy as recommended by vaccination schedules worldwide [14].
Data on the immunogenicity of hepatitis B vaccine in pediatric patients with IBD are limited. The results of previous studies reported that between 28% and 70.2% of children with IBD are seroprotected against HBV infection [10, 11]. However, many differences in the methodologies of these studies hamper the comparison of the results. First, we measured the antibody level approximately 14 years after vaccination. In the Urganci et al. study [12], the period after vaccination was 4 weeks, and the time was not reported in studies by Watts et al. [10] and Moses et al. [11]. It is documented that in immunocompetent individuals who are fully vaccinated against HBV, protective serologic titers may persist for more than 20 years, and no booster dose is recommended [15]. However, in immunosuppressed patients such as patients with IBD, the velocity of antibody weaning is unknown. Second, the dose of hepatitis B vaccine varies in pediatric studies. Urganci et al. [12] administered 20 µg hepatitis B vaccine, while three other studies, including ours, used a 10-µg dose [10, 11]. In all studies, full HBV vaccination was defined as 3 doses; however, in a study by Moses et al., “three or more doses” were considered as the full series [11]. Finally, the mean age of patients varied between 11 and almost 18 years. In conclusion, the cited studies are too different to summarize their results. In the adult IBD population, the results of a systematic review and meta-analysis of 13 studies assessing the immune response to hepatitis B vaccine revealed that the pooled rate of response was 61% (95% CI: 53-69%), which is similar to the result we have obtained [9].
In our study, we did not find any demographic or clinical factors associated with a positive response to vaccination against HBV. The results of other pediatric studies are divergent. Urganci et al., in their study, did not reveal any associations between age, sex, body-mass index, type of disease, or response rate [12]. In contrast, two other pediatric studies found that younger age was associated with higher vaccine response rate [10, 11]. In their meta-analysis, Jiang et al. found that sex, type of disease, disease duration, and comorbidities were not associated with the response to HBV vaccination [9]. However, they found age and disease activity to be factors that may modify the response. In 5 of 13 studies that assessed age as a variable for response to vaccination, young age was significantly associated with a higher response rate (MD, –5.7; 95% CI: –8.46, –2.95) [9]. We can speculate that younger age may be associated with shorter disease duration and shorter time of therapy; however, in none of those studies such analyses were performed. More studies are needed to explain the extent to which age influences the antibody response to hepatitis B vaccination.
We did not find any association between type of treatment and immune response to the hepatitis B vaccine. Our results are consistent with all previous pediatric studies [10-12]. In their systematic review with meta-analysis, Jiang et al. confirmed previous reports that adults with IBD not on immunosuppressive therapy had a higher immune response rate than patients on immunomodulators (RR, 1.33; 95% CI: 1.08-1.63) or on anti-TNF therapy (RR, 1.57; 95% CI: 1.19-2.08) [9]. The most probable explanation for this difference is the fact that children are likely to be treated with immunomodulators and/or biologicals for a much shorter time than adults. As an example, the disease duration, defined as the time from the date of IBD diagnosis to the date of the study entry, was 13 months in our study, 3.5 years in the Urganci et al. study, and 5.2 years in the Moses et al. study, compared to 5.9 years [16], 7.9 years [17], and 10.3 years in adult studies [18].
In our study, after the booster dose of hepatitis B vaccine, 41 of 45 (91%) patients with anti-HB levels < 10 mIU/ml achieved an adequate immune response. This is a much higher response rate than in the Urganci et al. study (7/14, 50% of patients) [12] or in the Moses et al. study (26/34, 76% of children) [11]. The difference may be a result of a longer serologic evaluation time: 8 weeks in our study compared to 4 weeks in the two other studies [10, 11]. Typically, it takes a few weeks for the human organism to produce T lymphocytes and B lymphocytes after vaccination. All patients who did not respond to the first booster dose of vaccine are being treated with immunomodulatory monotherapy; however, in this phase of the study, the four study groups were too small to conclude that immunomodulators suppress the hepatitis B vaccine response.
In a healthy population, it is well established that anti-HBs levels ≥ 10 mIU/ml are protective [5]. For immunocompromised adult individuals, it is strongly suggested that anti-HBs levels ≥ 100 mIU/ml are protective [14]. Therefore, we intended to verify how many of our patients achieved these high levels of anti-HBs. In phase 1 of the study, only 19.1% of them were highly immunized, but all others responded to additional doses of the vaccine (Fig. 2). We cannot compare our results with pediatric IBD studies because none of them assessed antibody levels ≥ 100 mIU/ml. To date, such an assessment has been performed in only a few studies on adult population with IBD. In three of them, patients were vaccinated against hepatitis B following a standard 3 dose protocol [19-21]. In these studies, the portion of population with anti-HBs levels ≥ 100 mIU/ml ranged from 31% to 53% of patients when measured 8 weeks after the third dose of the vaccine. Gisbert et al. found protective levels in 39% of patients vaccinated with one double dose (40 µg of HBsAg) of the vaccine and in 65% of patients vaccinated with 3 double doses of the vaccine [13]. The results of these studies are inconsistent; one of them [19] reported an association between a lower rate of seroconversion and older age irrespective of the fact that the mean ages of patients were similar (approximately 45 years) in all four studies. Waning of antibodies could explain the difference between the results of our study and those of adult studies (19.1% vs. 31%-53%, respectively); we assessed the levels of antibodies approximately 14 years after vaccination compared to the 8 weeks after vaccination cited in adult studies. Importantly, all our patients achieved an effective immune response after 3 doses of vaccine, whereas such a response was found in only 31-53% of adults. We can speculate that the duration of IBD therapy, not the type of therapy, or not only the type of therapy, may be a main risk factor for the lower response to the vaccine in adults. Therefore, the results of our study underline the importance of hepatitis B vaccination before IBD diagnosis or as early as possible after diagnosis. Moreover, it seems that patients with IBD must be “exposed to enough HBV antigens” to produce an effective number of antibodies. There were 3 additional doses of vaccine in our study and 3 double doses of vaccine in the Gisbert et al. study. We can expect that the response to wild HBV would be much stronger; therefore, we presume that the real protection against HBV is much greater than we can show using the booster dose(s) of hepatitis B vaccine.
The main advantage of this study is that for the first time, the effect of booster doses of hepatitis B vaccine in children with IBD was prospectively investigated. We assessed adequate (≥ 10 mIU/ml) and effective (≥ 100 mIU/ml) levels of anti-HBs. A similar study has been previously performed in adults with IBD, but not in a pediatric population. As a result, we found that all children with IBD achieved an effective response when we administered a 3-dose scheme of hepatitis B booster vaccination according to current IBD immunization recommendations. The study was conducted in Poland, but we can extrapolate our results to other countries of low endemicity for HBV, such as Europe and North America. The impact of our study is global, as both European and American immunization schedules recommend vaccination against hepatitis B in infancy. All medical associations focusing on IBD underline that hepatitis B vaccination is one of the most important vaccines for this group of patients. A shortcoming of this study is the lack of data on the duration of immunosuppressive treatment. Although we noted that the immunosuppression group included only patients who were treated with immunosuppressive therapy for 6 months or longer, we cannot assess the real impact of long-term immunosuppressive treatment on anti-HB waning. We had a high drop-out rate in the second part of the study; majority of them (20/28) did not give a reason of withdrawal. We think that this may be also a result of anti-vaccination movement affecting people in Europe. Additionally, our study did not include a control group; however, it is common to treat patients not on immunosuppressive therapy as controls [10, 11, 22, 23].
Conclusions
In conclusion, we found that the immune response against HBV was inadequate in almost half of the children with IBD who received full vaccination in infancy. However, induction of anti-HBs production by additional doses of the hepatitis B vaccine was highly effective if the patients were provided with a sufficient number of vaccine doses. Follow-up study that assess the waning antibody in this group of patients is required.